Combined risk of diabetes 1 272. High risk of Down syndrome, analysis and screening. Learn more on this topic
Greetings! If you remember the day when you or your child was diagnosed with diabetes, you will also remember the questions that began to worry your fevered brain. I dare to assume that you never received an answer to the question: “Where did type 1 diabetes mellitus come from, if there was no one in your family with this disease?”, just like to the question: “Is type 1 diabetes mellitus inherited and /or what will happen to the rest of the children and family members?” They probably still bother you to this day.
Today I will try to answer these questions. Type 1 diabetes is a multifactorial and polygenic disease. You can never say which factor is leading or main. Some scientists divide type 1 diabetes into subtypes: A and B. By the way, type 1 diabetes is not the only form that can occur in the younger generation. If you read the article ““, then you will learn more about this problem
Subtype A is associated with autoimmune damage to the pancreas and the detection of antibodies confirms this. This subtype is most often detected in children and adolescents. But it happens that antibodies are not detected, but diabetes is present. In this case, we are talking about subtype B, which occurs for completely different reasons not related to the functioning of the immune system. To date, these causes are not known, and therefore diabetes is called idiopathic.
Genetic testing for type 1 diabetes
One thing is clear that type 1 is a disease with a hereditary predisposition. What does this mean and how does it differ from just a hereditary disease? The fact is that a hereditary disease is the transmission of a gene from generation to generation or a mutation of a gene in a future organism. In this case, a new person is already born with a pathology or some other defect.
In the case of diabetes, everything is more complicated. There are certain genes and gene sections (I will speak in simplified terms), which, when combined in a certain way during the meeting of the egg and the sperm, increase the risk of developing type 1 diabetes. In other words, it is not the defective gene that is inherited, but the degree of risk for the disease. And for the disease to materialize, that is, to develop, provoking factors and a high degree of risk are necessary. If you conduct a genetic study, you can identify a certain degree of risk, which can be high, medium and low. Therefore, it is not at all necessary that if a person is at risk of developing type 1 diabetes, he will get it. Most often, the development of diabetes is associated with the following genes or gene regions - HLA DR3, DR4 and DQ.
In this regard, it does not matter at all if you have no known history of type 1 diabetes in your family now or in past generations. It is entirely possible that your ancestors had a low risk that never materialized. And besides this, how well do you know your family tree? What did children and adults die from at a young age? After all, diagnostics 100 years ago were not the most progressive, and doctors were not often consulted, especially in rural areas.
Therefore, I believe that it is completely pointless to look for those responsible for the spread of diabetes. Moreover, you should not reproach yourself (I am addressing the parents) for missing, not watching and not saving the child. To ease your guilt, I will say that the autoimmune process occurs long before the clinical manifestations of diabetes, about several years, and in some cases, ten years. Since then, a lot of water has flowed under the bridge and it is difficult to remember who is to blame for what. In the end, no matter how much we want, we cannot protect ourselves or our children from everything bad. Bad things happen and if this happened, then let's think that this is FATE, which cannot be deceived.
Immunoassay for type 1 diabetes mellitus
When a family has a relative with type 1 diabetes, then to predict the incidence of diabetes in other family members, not only a genetic study is used, but also the determination of autoantibodies, i.e. antibodies that fight against the tissues of one’s own body. For example, if an older child has type 1 diabetes, then parents can conduct genetic and antibody testing on the younger child to identify the risks of developing diabetes, because antibodies appear long before obvious ones.
- antibodies to islet beta cells - ICA (detected in 60-80% of cases) When combined with GAD, it sharply increases the risk of developing diabetes, but in isolation the risk of diabetes is small.
- anti-insulin antibodies - IAA (detected in 30-60% of cases) In isolated form, has little effect on the development of diabetes; the risk increases in the presence of any other antibodies.
- antibodies to glutamate decarboxylase - GAD (detected in 80-95% of cases) Increases the risk of developing diabetes, even in isolated form.
But here, too, everything is ambiguous. The detection of any one group of antibodies in a child does not mean at all that he will develop diabetes in the future. This only means that this child has a high risk of developing diabetes, which may not materialize. And then, no one is safe from a laboratory error, so it is recommended to retake the tests after 1-2 months.
Therefore, I do not recommend testing for antibodies in healthy family members. IMHO. What can you do if you know you have antibodies? Of course, you can get into experimental groups where they test methods for preventing diabetes in high-risk groups, but would you want to subject a still healthy child to unknown manipulations? Personally, I’m not ready, and we live far from the center of the country.
Apart from unnecessary hassle, these actions do not bring anything good. Constant expectations and thoughts may one day come true. Personally, I believe that our thoughts are material and everything we think about will someday come true. Therefore, you don’t need to think about the bad, attract only positive thoughts that everything will be fine and all other family members will be healthy. The only thing that can be done is to periodically determine fasting glucose and/or glycated hemoglobin so as not to miss the manifestation of diabetes. Because so far there are no proven methods that 100% prevent the development of diabetes, and there are none at all.
Another question that concerns everyone with type 1 diabetes: “What are the risks of illness in children whose parents have diabetes or if there is already a child with diabetes in the family?” A 16-year study was recently completed that examined the prognosis of the disease in the families of patients. Here are his results.
The risk of developing diabetes without a known relative with diabetes is only 0.2 – 0.4%. The greater the number of relatives with diabetes in a family, the higher the risk. The risk of developing diabetes for family members of a person with type 1 diabetes is on average 5%. If two children in a family are sick, the risk for the third is 9.5%. If two parents are sick, then the risk of developing type 1 diabetes for the child already increases to 34%. In addition, the risk of developing type 1 diabetes depends on the age at which the disease manifests itself in the patient. The earlier a child in the family gets sick, the higher the risk for the second one. If the manifestation of the disease occurred before the age of 20, then the risk for the second child is 6.4%, and if the manifestation of the disease is older than 20 years, then the risk is 1.2%.
Prevention of type 1 diabetes
But what can be done to reduce the influence of these notorious factors that trigger the autoimmune process? And although it all comes down to “lucky or unlucky,” you can still try to influence them as much as possible. Here is a list of methods for the primary prevention of type 1 diabetes.
- Prevention of intrauterine infection and viral infections of the mother during pregnancy.
- Prevention of certain viral infections in children and adolescents, such as rubella, measles, mumps, enteroviruses, chicken pox, influenza.
- Timely treatment of chronic foci of infection (sinusitis, carious teeth, etc.).
- Carrying out timely vaccination, strictly according to the rules and proven vaccines.
- Excluding cow's milk protein from the diet of infants.
- Long-term breastfeeding (minimum 18 months).
- Excluding the introduction of complementary foods containing gluten-containing products under the age of one year.
- Exclusion from the diet of foods containing nitrates, preservatives and dyes.
- Normal intake of vitamin D.
- Adding Omega 3 fatty acid supplements to your diet.
- Reducing the consumption of fast carbohydrates due to excessive stress on the pancreas.
In conclusion, I want to say. We are all different, with varying degrees of anxiety and “not giving a fuck.” Therefore, it’s up to you to decide whether to have your child diagnosed with diabetes or go yourself. Ask yourself: “Are you ready for a positive outcome? Are you ready to find out that your child is at risk of developing this disease and at the same time continue to live peacefully?” If yes, then you can undergo a complete genetic and immune examination. It is best to do this in the very heart of the country and endocrinology - the Endocrinological Scientific Center in Moscow.
I end here and sincerely wish healthy people to avoid the “delights” of type 1 diabetes. Until next time.
With warmth and care, endocrinologist Lebedeva Dilyara Ilgizovna
Diabetes mellitus is a complex disease that is difficult to treat. When it develops in the body, carbohydrate metabolism is disrupted and insulin synthesis by the pancreas is reduced, as a result of which glucose ceases to be absorbed by cells and settles in the blood in the form of microcrystalline elements.
Scientists have still not been able to establish the exact reasons why this disease begins to develop. But they identified risk factors for diabetes mellitus that can trigger the onset of this disease in both older and younger people.
A few words about pathology
Before considering the risk factors for developing diabetes, it must be said that this disease has two types, and each of them has its own characteristics. Type 1 diabetes is characterized by systemic changes in the body, in which not only carbohydrate metabolism is disrupted, but also the functionality of the pancreas. For some reason, its cells stop producing insulin in the required amount, as a result of which the sugar that enters the body along with food does not undergo breakdown processes and, accordingly, cannot be absorbed by the cells.
Type 2 diabetes mellitus is a disease in which the functionality of the pancreas is preserved, but due to impaired metabolism, the body's cells lose sensitivity to insulin. Against this background, glucose simply stops being transported into cells and settles in the blood.
But no matter what processes occur during diabetes, the result of this disease is the same - high levels of glucose in the blood, which leads to serious health problems.
The most common complications of this disease are the following conditions:
- hyperglycemia – an increase in blood sugar levels beyond normal limits (over 7 mmol/l);
- hypoglycemia – a decrease in blood glucose levels beyond normal limits (below 3.3 mmol/l);
- hyperglycemic coma – increased blood sugar levels over 30 mmol/l;
- hypoglycemic coma – a decrease in blood glucose levels below 2.1 mmol/l;
- diabetic foot – decreased sensitivity of the lower extremities and their deformation;
- diabetic retinopathy – decreased visual acuity;
- thrombophlebitis - the formation of plaques in the walls of blood vessels;
- hypertension - increased blood pressure;
- gangrene – necrosis of tissues of the lower extremities with subsequent development of an abscess;
- stroke and myocardial infarction.

Common complications of diabetes
These are not all the complications that the development of diabetes mellitus poses to a person at any age. And in order to prevent this disease, it is necessary to know exactly what factors can provoke the occurrence of diabetes and what measures include prevention of its development.
Type 1 diabetes mellitus and its risk factors
Type 1 diabetes mellitus (T1DM) is most often diagnosed in children and young people aged 20-30 years. It is believed that the main factors of its development are:
- viral diseases;
- intoxication of the body;
- poor nutrition;
- frequent stress.
Hereditary predisposition plays a major role in the occurrence of T1DM. If one of the family members suffers from this disease, then the risk of its development in the next generation is approximately 10-20%.
It should be noted that in this case we are not talking about an established fact, but about a predisposition. That is, if a mother or father has T1DM, this does not mean that their children will also be diagnosed with this disease. Predisposition suggests that if a person does not carry out preventive measures and leads an incorrect lifestyle, then he has a high risk of becoming diabetic within several years.

When both parents are diagnosed with diabetes at once, the risk of developing the disease in their children increases several times.
However, even in this case, it is necessary to take into account that if both parents suffer from diabetes, then the likelihood of it developing in their child increases significantly. And it is often in such situations that this disease is diagnosed in children at school age, although they do not yet have bad habits and lead an active lifestyle.
It is believed that diabetes mellitus is most often “transmitted” through the male line. But if only the mother has diabetes, then the risks of giving birth to a baby with this disease are very low (no more than 10%).
Viral diseases
Viral diseases are another reason why T1DM can develop. Particularly dangerous in this case are diseases such as mumps and rubella. Scientists have long proven that these diseases negatively affect the functioning of the pancreas and lead to damage to its cells, thus reducing the level of insulin in the blood.
It should be noted that this applies not only to already born children, but also to those who are still in the womb. Any viral diseases that a pregnant woman suffers can trigger the development of T1DM in her child.
Intoxication of the body
Many people work in factories and enterprises where chemicals are used, the effects of which negatively affect the functioning of the entire body, including the functionality of the pancreas.
Chemotherapy, which is carried out to treat various oncological diseases, also has a toxic effect on the body’s cells, so their use also increases the likelihood of developing T1DM in a person several times.
Poor nutrition
Poor nutrition is one of the most common causes of T1DM. The daily diet of a modern person contains a huge amount of fats and carbohydrates, which puts a strong burden on the digestive system, including the pancreas. Over time, its cells are damaged and insulin synthesis is disrupted.

Poor nutrition is dangerous not only for the development of obesity, but also for disruption of the pancreas.
It should also be noted that due to poor nutrition, T1DM can also develop in children aged 1-2 years. And the reason for this is the early introduction of cow’s milk and cereals into the baby’s diet.
Frequent stress
Stress is a provocateur of various diseases, including T1DM. If a person experiences stress, his body produces a lot of adrenaline, which promotes the rapid processing of sugar in the blood, resulting in hypoglycemia. This condition is temporary, but if it occurs systematically, the risk of developing T1DM increases several times.
Type 2 diabetes mellitus and its risk factors
As mentioned above, type 2 diabetes mellitus (T2DM) develops as a result of decreased sensitivity of cells to insulin. This can also happen for several reasons:
- hereditary predisposition;
- age-related changes in the body;
- obesity;
- gestational diabetes.
Hereditary predisposition
In the development of T2DM, hereditary predisposition plays an even greater role than in T1DM. Statistics show that the risk of developing this disease in offspring in this case is 50% if T2DM was diagnosed only in the mother, and 80% if this disease was diagnosed in both parents at once.

When parents are diagnosed with T2DM, the likelihood of having a sick child is significantly higher than with T1DM
Age-related changes in the body
Doctors consider T2DM a disease of older people, since it is in them that it is most often detected. The reason for this is age-related changes in the body. Unfortunately, with age, under the influence of internal and external factors, internal organs “wear out” and their functionality is impaired. In addition, as many people age, hypertension develops, which further increases the risk of developing T2DM.
Important! In view of all this, doctors highly recommend that all people over 50 years of age, regardless of their general health and gender, regularly take tests to determine their blood sugar levels. And if any abnormalities are detected, begin treatment immediately.
Obesity is the main cause of T2DM in both older and younger people. The reason for this is the excess accumulation of fat in the cells of the body, as a result of which they begin to draw energy from it, and sugar becomes unnecessary for them. Therefore, with obesity, cells stop absorbing glucose, and it settles in the blood. And if a person, who is overweight, also leads a passive lifestyle, this further increases the likelihood of developing T2DM at any age.

Obesity provokes the appearance of not only T2DM, but also other health problems
Gestational diabetes
Gestational diabetes is also called “pregnant diabetes” by doctors, as it develops during pregnancy. Its occurrence is caused by hormonal disorders in the body and excessive activity of the pancreas (it has to work for “two”). Due to increased stress, it wears out and stops producing insulin in the required quantities.
After childbirth, this disease goes away, but leaves a serious mark on the child’s health. Due to the fact that the mother's pancreas stops producing insulin in the required amount, the child's pancreas begins to work at an accelerated rate, which leads to damage to its cells. In addition, with the development of gestational diabetes, the risk of obesity in the fetus increases, which also increases the risk of developing T2DM.
Prevention
Diabetes mellitus is a disease whose development can be easily prevented. To do this, it is enough to constantly carry out its prevention, which includes the following measures:
- Proper nutrition. Human nutrition should include many vitamins, minerals and proteins. Fats and carbohydrates should also be present in the diet, since without them the body cannot function normally, but in moderation. You should especially beware of easily digestible carbohydrates and trans fats, since they are the main cause of excess body weight and the further development of diabetes. As for infants, parents should ensure that the complementary foods introduced are as beneficial as possible for their body. You can find out what you can give your baby and in what month from your pediatrician.
- Active lifestyle. If you neglect sports and lead a passive lifestyle, you can also easily “earn” diabetes. Human activity promotes rapid fat burning and energy consumption, resulting in an increased cell need for glucose. In passive people, metabolism slows down, resulting in an increased risk of developing diabetes.
- Monitor your blood sugar levels regularly. This rule especially applies to those who have a hereditary predisposition to this disease, and people who have turned 50 years old. To monitor your blood sugar levels, it is not at all necessary to constantly go to the clinic and get tested. You just need to purchase a glucometer and conduct blood tests yourself at home.
It should be understood that diabetes is a disease that cannot be cured. When it develops, you have to constantly take medications and take insulin injections. Therefore, if you do not want to always be in fear for your health, lead a healthy lifestyle and promptly treat any diseases that arise. This is the only way to prevent the occurrence of diabetes and maintain your health for many years!
Last updated: April 18, 2018
Down syndrome is not a disease, it is a pathology that cannot be prevented or cured. A fetus with Down syndrome has a third extra chromosome on the 21st pair of chromosomes, resulting in a total of 47 instead of 46. Down syndrome occurs in one in 600-1000 newborns born to women over the age of 35. The reason why this happens , has not been fully clarified. English physician John Langdon Down first described this syndrome in 1866, and in 1959 French professor Lejeune proved that it is associated with genetic changes.
It is known that children receive half of their chromosomes from their mother, and half from their father. Since there is not a single effective method of treating Down syndrome, the disease is considered incurable, you can take action and, if you want to give birth to a healthy child, go to a medical genetic consultation, where, based on the chromosomal analysis of the parents, it will be determined whether the child will be born healthy or with Down syndrome.
Recently, such children are born more often; this is associated with late marriage, with planning a pregnancy at the age of 40. It is also believed that if a grandmother gave birth to her daughter after 35, then her grandchildren may be born with Down syndrome. Although prenatal diagnosis is a complex examination process, its implementation is very necessary in order to be able to terminate the pregnancy.
What is Down syndrome? It may usually be accompanied by delayed motor development. Such children have congenital heart defects and pathological development of the gastrointestinal tract. 8% of patients with Down syndrome have leukemia. Drug treatment can stimulate mental activity and normalize hormonal imbalance. With the help of physiotherapeutic procedures, massage, and therapeutic exercises, you can help your child acquire the skills necessary for self-care. Down syndrome is associated with a genetic disorder, but this does not always lead to impaired physical and mental development of the child. Such children, and in the future adults, can participate in all walks of life, some of them become actors, athletes and can be involved in public affairs. How a person with this diagnosis will develop depends largely on the environment in which he grows up. Good conditions, love and care contribute to full development.
Down syndrome risk table by age
The likelihood of Down syndrome depends on the age of the mother, but it can be detected by a genetic test in the early stages of pregnancy and, in some cases, ultrasound. The baby is less likely to have Down syndrome at birth than in earlier stages of pregnancy because some fetuses with Down syndrome do not survive.

What risk is considered low and what is considered high?
In Israel, the risk of Down syndrome is considered high if it is higher than 1:380 (0.26%). Anyone who is in this risk group needs to have their amniotic fluid tested. This risk is equivalent to that of women who become pregnant at age 35 or older.
A risk lower than 1:380 is considered low.
But we must take into account that these boundaries can be floating! For example, in England, a high risk level is considered to be a risk above 1:200 (0.5%). This happens because some women consider a risk of 1 in 1000 to be high, while others consider a risk of 1 in 100 to be low, since with such a risk they have a 99% chance of having a healthy child.
Risk factors for Down syndrome, Edwards syndrome, Patau syndrome
The main risk factors are age (especially significant for Down syndrome), as well as exposure to radiation and certain heavy metals. It should be borne in mind that even without risk factors, the fetus can have pathology.
As can be seen from the graph, the dependence of the risk on age is most significant for Down syndrome, and less significant for the other two trisomies:

Down syndrome risk screening
Today, all pregnant women, in addition to the required tests, are recommended to undergo a screening test to determine the degree of risk of Down syndrome due to the birth of a child and congenital defects of the fetus. The most productive examination occurs at week 11 + 1 day or at week 13 + 6 days when the coccygeal-parietal size of the embryo is from 45 mm to 84 mm. A pregnant woman can undergo an examination and use a specific ultrasound for this.
A more accurate diagnosis is made using chorionic villus biopsy and examination of amniotic fluid, which is taken directly from the amniotic sac using a special needle. But every woman should know that such methods are associated with the risk of pregnancy complications such as miscarriage, infection of the fetus, development of hearing loss in the child, and much more.
Complete combined screening of the first and second trimesters of pregnancy allows us to identify congenital defects in the fetus. What does this test include? First, an ultrasound examination is required at 10-13 weeks of pregnancy. The risk is calculated by determining the presence of the nasal bone and the width of the fetal neck fold, where subcutaneous fluid accumulates in the first trimester of pregnancy.
In the second, a blood test is taken for human chorionic gonadotropin at 10-13 weeks and for alpha-fetoprotein at 16-18 weeks. Combined screening data is processed using a special computer program. Scientists have proposed a new screening technique - combining the assessment of results obtained during studies in the first and second trimesters. This allows for a unified assessment of the risk of Down syndrome during pregnancy.
For the first trimester, the results of determining PAPP-A and measuring the thickness of the nuchal translucency are used, and for the second trimester, combinations of AFP, unconjugated estriol, hCG and inhibin-A are used. The use of an integral assessment for screening examination allows, after invasive interventions, to reduce the rate of abortion for fetuses with a normal karyotype according to the results of cytogenetic diagnostics.
Integral and biochemical testing for Down syndrome screening can further identify more cases of chromosomal abnormalities. This helps prevent unwanted pregnancy losses resulting from amniocentesis or chorionic villus sampling.
Expert editor: Mochalov Pavel Alexandrovich| Doctor of Medical Sciences therapist
Education: Moscow Medical Institute named after. I. M. Sechenov, specialty - "General Medicine" in 1991, in 1993 "Occupational diseases", in 1996 "Therapy".
Hi all! Girls who have been in similar situations, please respond! On May 27th I had my first screening. The ultrasound showed everything was normal. They wrote down the phone number just in case, but I didn’t expect that they might call me back, and then a week later I got a call - come for a referral to the Center for Psychological Surveillance, you are at high risk. I don’t remember myself, I arrived in tears, on weak legs, and took all the papers. Risk 1:53. The next day I went for further examination. The ultrasound specialist looked at both the abdomen and vagina for a very long time, turned on the Doppler several times, and everything seemed to be fine, but he didn’t like DOPPLER METRY OF THE TRISCUPID VALVE: REGURGITATION. I entered the new ultrasound data into the program and the screening results from a week ago, the computer showed a diabetes risk of 1:6. I sent him to a geneticist. After looking at the conclusion, she explained to me that this regurgitation could simply be a feature of the fetus, but coupled with an underestimated PAPP-A indicator - 0.232 MoM, this is a marker of chromosomal abnormalities. Everything else is within normal limits. They suggested undergoing a chorionic villus biopsy. I refused for now, the nurse almost fell out of her chair, like the risk is so high and CA cannot be treated, and if she were me, she wouldn’t even think for a minute. I asked a geneticist about the Panorama analysis (a terribly expensive genetic analysis of maternal blood), she told me that of course you can do it, but it excludes only 5 main CAs and several very rare ones, it cannot completely exclude anomalies, and in my case it is recommended invasion. I’ve already read a ton of articles, questions and the like on this topic, and I just don’t understand what they found so terrible in my analyzes? Regurgitation, as it turned out, is physiological at this stage and goes away by 18-20 weeks (if it doesn’t go away, this indicates a risk of heart defects, for many it goes away after childbirth, and some live with it and doesn’t affect anything. Moreover, the husband has prolapse mintral valve, which was inherited from my mother, maybe this is somehow connected). Hormones may not be indicative at all, because... I’ve been taking Duphaston since the beginning of pregnancy, I ate 2 hours before the test (it turns out you can’t eat 4 hours before, they didn’t tell me about this), drank coffee, was nervous and worried about the ultrasound and I’m afraid to donate blood, and lately I’ve had chronic fatigue , I’m tired with my older child. And all this affects the results. The geneticist didn’t ask anything of the kind, wasn’t interested, they actually have some kind of conveyor belt there, and it was as if they shoved me there for statistics. But they planted a bit of doubt in me, I cried and was not worried about the year ahead. My husband is trying to persuade me to have a biopsy. I am terribly afraid of the consequences, afraid of losing or harming the child, especially if he is healthy. On the one hand, if everything is fine, I will breathe a sigh of relief and send all the doctors away. On the other hand, if everything is bad, what should you do? Will I be able to terminate the pregnancy, allow my child to be dismembered inside me, especially now that it seems to me that I am beginning to feel him. But another option is whether I can raise a child who requires a special approach and a lot of attention, when sometimes I want to run away from a completely healthy daughter... Damn, all these thoughts are eating me up. I don’t know what to do... Just in case, I’ll give you the screening data:
Delivery period: 13 weeks
Heart rate 161 beats/min
Ductus venosus PI 1.160
Chorion/placenta low on the anterior wall
Umbilical cord 3 vessels
Fetal anatomy: everything is determined, everything is normal
b-hCG 1.091 MoM
PAPP-A 0.232 MoM
Uterine artery PI 1,240 MoM
Trisomy 21 1:6
Trisomy 18 1:311
Trisomy 13 1:205
Preeclampsia up to 34 weeks 1:529
Preeclampsia up to 37 weeks 1:524
7.1. CLASSIFICATION OF DIABETES MELLITUS
Diabetes(DM) is a group of metabolic diseases characterized by hyperglycemia due to impaired secretion and/or efficiency of insulin action. Chronic hyperglycemia, which develops with diabetes, is accompanied by the development of complications from many organs and systems, primarily from the heart, blood vessels, eyes, kidneys and nerves. In total, 5-6% of the population suffers from diabetes. In economically developed countries of the world, every 10-15 years the number of patients with diabetes doubles. Life expectancy with diabetes is reduced by 10-15%.
The causes of diabetes vary widely. In the vast majority of cases, diabetes develops either due to an absolute deficiency of insulin (diabetes mellitus type 1 - DM-1), or due to decreased sensitivity of peripheral tissues to insulin in combination with secretory dysfunction of pancreatic β-cells (diabetes mellitus type 2 - SD-2). In some cases, classifying a patient as DM-1 or DM-2 is difficult; however, in practice, compensation for DM is more important, rather than accurately identifying its type. The etiological classification identifies four main clinical classes of diabetes (Table 7.1).
The most common DM-1 (clause 7.5), DM-2 (clause 7.6) and gestational DM (clause 7.9) are discussed in separate chapters. On other specific types accounts for only about 1% of diabetes cases. The etiology and pathogenesis of these types of diabetes seems to be more studied compared to diabetes 1 and especially diabetes 2. A number of DM variants are caused by monogenically inherited genetic defects in functionβ -cells. This includes various variants of the autosomal dominantly inherited MODY syndrome. maturity onset diabetes of the young- diabetes of the adult type in young people), which are characterized by a violation, but not the absence of insulin secretion with normal sensitivity of peripheral tissues to it.
Table 7.1. Classification of diabetes mellitus
Casuistically rare genetic defects in insulin action, associated with a mutation of the insulin receptor (leprechaunism, Rabson-Mandehall syndrome). DM naturally develops with diseases of the exocrine pancreas, leading to the destruction of β-cells (pancreatitis, pancreatectomy, cystic fibrosis, hemochromatosis), as well as in a number of endocrine diseases in which excessive production of counterinsular hormones occurs (acromegaly, Cushing's syndrome). Medicines and chemicals(vacor, pentamidine, nicotinic acid, diazoxide, etc.) rarely cause diabetes, but can contribute to the manifestation and decompensation of the disease in individuals with insulin resistance. Row infectious diseases(rubella, cytomegaly, coxsackievirus and adenovirus infections) may be accompanied by destruction of β-cells, while immunogenetic markers of DM-1 are detected in most patients. TO rare forms of immune-mediated diabetes include diabetes developing in patients with “stiff-rnan” syndrome (an autoimmune neurological disease), as well as diabetes due to exposure to autoantibodies to insulin receptors. Various variants of diabetes with increased frequency occur in
many genetic syndromes, in particular, Down syndrome, Klinefelter syndrome, Turner syndrome, Wolfram syndrome, Prader-Willi syndrome and a number of others.
7.2. CLINICAL ASPECTS OF THE PHYSIOLOGY OF CARBOHYDRATE METABOLISM
Insulin synthesized and secreted by β-cells of the islets of Langerhans of the pancreas (PLI). In addition, the islets of Langerhans secrete glucagon (α cells), somatostatin (δ cells) and pancreatic polypeptide (PP cells). Islet cell hormones interact with each other: glucagon normally stimulates insulin secretion, and somatostatin suppresses the secretion of insulin and glucagon. The insulin molecule consists of two polypeptide chains (A chain - 21 amino acids; B chain - 30 amino acids) (Fig. 7.1). Insulin synthesis begins with the formation of preproinsulin, which is cleaved by protease to form proinsulin. In the secretory granules of the Golgi apparatus, proinsulin is broken down into insulin and C-peptide, which are released into the blood during the process of exocytosis (Fig. 7.2).
The main stimulator of insulin secretion is glucose. Insulin is released in response to increased blood glucose levels two-phase(Fig. 7.3). The first, or acute, phase lasts several minutes, and is associated with the release of accumulated
 Rice. 7.1. Diagram of the primary structure of the insulin molecule
Rice. 7.1. Diagram of the primary structure of the insulin molecule
 Rice. 7.2. Insulin biosynthesis scheme
Rice. 7.2. Insulin biosynthesis scheme
insulin present in the β-cell during the period between meals. The second phase continues until the glycemic level reaches normal fasting levels (3.3-5.5 mmol/l). Sulfonylureas have a similar effect on the β-cell.
Through the portal system, insulin reaches liver- its main target organ. Liver receptors bind half of the secreted hormone. The other half, entering the systemic circulation, reaches muscles and adipose tissue. Most insulin (80%) undergoes proteolytic breakdown in the liver, the rest in the kidneys, and only a small amount is metabolized directly by muscle and fat cells. Lifespan is normal
 Rice. 7.3. Biphasic release of insulin under the influence of glucose
Rice. 7.3. Biphasic release of insulin under the influence of glucose
an adult person secretes 35-50 units of insulin per day, which is 0.6-1.2 units per 1 kg of body weight. This secretion is divided into nutritional and basal. Food secretion insulin corresponds to a postprandial rise in glucose levels, i.e. due to it, the hyperglycemic effect of food is neutralized. The amount of dietary insulin approximately corresponds to the amount of carbohydrates taken - about 1-2.5 units
for 10-12 g of carbohydrates (1 bread unit - XE). Basal insulin secretion provides an optimal level of glycemia and anabolism in the intervals between meals and during sleep. Basal insulin is secreted at a rate of approximately 1 U/h; with prolonged physical activity or prolonged fasting, it decreases significantly. Dietary insulin accounts for at least 50-70% of daily insulin production (Fig. 7.4).
Insulin secretion is affected not only by food, but also daily
 Rice. 7 .4.
Normal daily insulin production pattern
Rice. 7 .4.
Normal daily insulin production pattern
ny fluctuations: The need for insulin increases in the early morning hours and then gradually decreases throughout the day. So, for breakfast per 1 XE 2.0-2.5 units of insulin are secreted, for lunch - 1.0-1.5 units, and for dinner - 1.0 units. One of the reasons for this change in insulin sensitivity is the high level of a number of counter-insular hormones (primarily cortisol) in the morning, which gradually drops to a minimum at the beginning of the night.
Main physiological effects of insulin are stimulation of glucose transfer across cell membranes of insulin-dependent tissues. The main target organs for insulin are the liver, adipose tissue and muscle. Insulin-independent tissues, the supply of glucose into which does not depend on the effects of insulin, primarily include the central and peripheral nervous system, vascular endothelium, blood cells, etc. Insulin stimulates the synthesis of glycogen in the liver and muscles, the synthesis of fats in the liver and adipose tissue, the synthesis proteins in the liver, muscles and other organs. All these changes are aimed at the utilization of glucose, which leads to a decrease in its level in the blood. A physiological antagonist of insulin is glucagon, which stimulates the mobilization of glycogen and fats from the depot; Normally, glucagon levels change reciprocally with insulin production.
The biological effects of insulin are mediated by its receptors which are located on target cells. The insulin receptor is a glycoprotein consisting of four subunits. With a high level of insulin in the blood, the number of its receptors decreases according to the principle of down regulation, which is accompanied by a decrease in the cell’s sensitivity to insulin. After insulin binds to the cellular receptor, the resulting complex enters the cell. Further inside muscle and fat cells, insulin causes the mobilization of intracellular vesicles that contain glucose transporter GLUT-4. As a result, the vesicles move to the cell surface, where GLUT-4 acts as an entry point for glucose. Physical activity has a similar effect on GLUT-4.
7.3. LABORATORY DIAGNOSTICS AND COMPENSATION CRITERIA FOR DIABETES MELLITUS
Laboratory diagnosis of diabetes is based on determining blood glucose levels, and the diagnostic criteria are the same for everyone
types and variants of SD (Table 7.2). Data from other laboratory tests (glucosuria level, determination of glycated hemoglobin level) should not be used to verify the diagnosis of diabetes. The diagnosis of diabetes can be established on the basis of double detection of one of three criteria:
1. With obvious symptoms of diabetes (polyuria, polydipsia) and the level of glucose in whole capillary blood is more than 11.1 mmol/l, regardless of the time of day and the previous meal.
2. When the glucose level in fasting whole capillary blood is more than 6.1 mmol/l.
3. When the glucose level in whole capillary blood 2 hours after taking 75 grams of glucose (oral glucose tolerance test) is more than 11.1 mmol/l.
Table 7.2. Criteria for diagnosing diabetes mellitus
 The most important and significant test in the diagnosis of diabetes is to determine the level of fasting glycemia (minimum 8 hours of fasting). In the Russian Federation, glycemic levels are usually assessed in whole blood. Glucose testing is widely used in many countries
The most important and significant test in the diagnosis of diabetes is to determine the level of fasting glycemia (minimum 8 hours of fasting). In the Russian Federation, glycemic levels are usually assessed in whole blood. Glucose testing is widely used in many countries
in blood plasma. Oral glucose tolerance test(OGTT; determination of glucose levels 2 hours after ingestion of 75 grams of glucose dissolved in water) is given less importance in this regard. However, based on the OGTT, it is diagnosed impaired glucose tolerance(NTG). IGT is diagnosed if the glucose level of fasting whole capillary blood does not exceed 6.1 mmol/l, and 2 hours after a glucose load it is above 7.8 mmol/l, but below 11.1 mmol/l. Another variant of carbohydrate metabolism disorder is impaired fasting glucose(NGNT). The latter is established if the level of glycemia of whole capillary blood on an empty stomach is in the range of 5.6-6.0 mmol/l, and 2 hours after a glucose load is less than 7.8 mmol/l). NTG and NGNT are currently combined under the term prediabetes, since both categories of patients have a high risk of manifesting diabetes and developing diabetic macroangiopathy.
To diagnose diabetes, glycemic levels must be determined by standard laboratory methods. When interpreting glycemic values, it should be borne in mind that the fasting level of glucose in whole venous blood corresponds to its level in whole capillary blood. After a meal or OGTT, its level in venous blood is approximately 1.1 mmol/l lower than in capillary blood. The glucose content in plasma is approximately 0.84 mmol/l higher than in whole blood. In order to assess compensation and adequacy of diabetes therapy, the level of glycemia is assessed in capillary blood using portable glucometers by the patients themselves, their relatives or medical staff.
With any type of diabetes, as well as with a significant glucose load, it can develop glucosuria, which is a consequence of exceeding the threshold for glucose reabsorption from primary urine. The threshold for glucose reabsorption varies significantly individually (≈ 9-10 mmol/l). Glucosuria should not be used as a separate indicator for diagnosing diabetes. Normally, except in cases of significant dietary load of refined carbohydrates, glucosuria does not occur.
Products ketone bodies(acetone, acetoacetate, β-hydroxybutyrate) is significantly intensified with absolute insulin deficiency. With decompensation of DM-1, pronounced ketonuria(tested using test strips that are dipped into urine). Mild (trace) ketonuria can be detected in healthy people during fasting and a carbohydrate-free diet.
An important laboratory indicator that is used for differential diagnosis of types of diabetes, as well as to identify the formation of insulin deficiency in patients with diabetes-2, is the level C-peptide. The level of C-peptide in the blood can indirectly judge the insulin-secreting ability of β-cells of the pancreas. The latter produce proinsulin, from which C-peptide is cleaved before secretion, entering the blood in equal quantities with insulin. Insulin is 50% bound in the liver and has a half-life in peripheral blood of about 4 minutes. C-peptide is not removed from the bloodstream by the liver and has a half-life in the blood of about 30 minutes. In addition, it does not bind to cellular receptors in the periphery. Therefore, determining the level of C-peptide is a more reliable test for assessing the function of the insular apparatus. It is most informative to study the level of C-peptide against the background of stimulation tests (after eating or administering glucagon). The test is not informative if it is performed against the background of severe decompensation of diabetes, since severe hyperglycemia has a toxic effect on β-cells (glucotoxicity). Insulin therapy over the previous few days will not affect the test results.
Basic purpose of treatment of any type of diabetes is the prevention of its late complications, which can be achieved against the background of its stable compensation for a number of parameters (Table 7.3). The main criterion for the quality of compensation of carbohydrate metabolism in diabetes is the level glycated (glycosylated) hemoglobin (HbA1c). The latter is hemoglobin non-covalently bound to glucose. Glucose enters erythrocytes independently of insulin, and glycosylation of hemoglobin is an irreversible process, and its degree is directly proportional to the concentration of glucose with which it was in contact during the 120 days of its existence. A small portion of hemoglobin is glycosylated and is normal; in diabetes it can be significantly increased. The HbA1c level, unlike the glucose level, which is constantly changing, integrally reflects glycemia over the past 3-4 months. It is at this interval that it is recommended to determine the HbA1c level in order to assess diabetes compensation.
Chronic hyperglycemia is far from the only risk factor for the development and progression of late complications of diabetes. Due to this DM compensation assessment based on a complex
laboratory and instrumental research methods (Table 7.3). In addition to indicators characterizing the state of carbohydrate metabolism, the most important are the level of blood pressure and the lipid spectrum of the blood.
Table 7.3. Criteria for compensation of diabetes mellitus
 In addition to the above compensation criteria, an individual approach is required when planning goals for treating diabetes. The likelihood of development and progression of late complications of diabetes (especially microangiopathy) increases with increasing disease duration. Thus, if in children and young patients, whose history of diabetes may subsequently reach several decades, it is necessary to achieve optimal glycemic indicators, then in patients in whom diabetes manifested itself in old age, strict euglycemic compensation, which significantly increases the risk of hypoglycemia, not always advisable.
In addition to the above compensation criteria, an individual approach is required when planning goals for treating diabetes. The likelihood of development and progression of late complications of diabetes (especially microangiopathy) increases with increasing disease duration. Thus, if in children and young patients, whose history of diabetes may subsequently reach several decades, it is necessary to achieve optimal glycemic indicators, then in patients in whom diabetes manifested itself in old age, strict euglycemic compensation, which significantly increases the risk of hypoglycemia, not always advisable.
7.4. INSULIN PREPARATIONS AND INSULIN THERAPY
Insulin preparations are vital for patients with type 1 diabetes; in addition, up to 40% of patients with T2DM receive them. To general indications for prescribing insulin therapy for diabetes, many of which actually overlap one another include:
1. Diabetes mellitus type 1
2. Pancreatectomy
3. Ketoacidotic and hyperosmolar coma
4. For type 2 diabetes mellitus:
Clear signs of insulin deficiency, such as progressive weight loss and ketosis, severe hyperglycemia;
Major surgical interventions;
Acute macrovascular complications (stroke, myocardial infarction, gangrene, etc.) and severe infectious diseases accompanied by decompensation of carbohydrate metabolism;
Fasting glucose level is more than 15-18 mmol/l;
Lack of stable compensation, despite the prescription of maximum daily doses of various tableted hypoglycemic drugs;
Late stages of late complications of diabetes (severe polyneuropathy and retinopathy, chronic renal failure).
5. Inability to achieve compensation for gestational diabetes with diet therapy.
By origin Insulin preparations can be classified into three groups:
Animal insulins (pork);
Human insulins (semi-synthetic, genetically engineered);
Insulin analogs (lispro, aspart, glargine, detemir).
Advances in human insulin production technology have led to the use of pork insulin(differs from human one in one amino acid) has recently decreased significantly. Porcine insulin could be used to produce human insulin semi-synthetic method, which involves replacing one different amino acid in its molecule. The highest quality genetic engineering human insulins. To obtain them, the region of the human genome responsible for insulin synthesis is associated with the genome E.coli or yeast culture, as a result of which the latter begin to produce human insulin. Creation insulin analogues using rearrangements of various amino acids, the goal was to obtain drugs with the desired and most favorable pharmacokinetics. Thus, insulin lispro (Humalog) is an analogue
ultra-short-acting insulin, with its hypoglycemic effect developing within 15 minutes after injection. The insulin analogue glargine (Lantus), on the contrary, is characterized by a long-term effect that lasts throughout the day, while a feature of the kinetics of the drug is the absence of pronounced peaks in plasma concentration. Most currently used insulin preparations and its analogues are produced in concentrations 100 U/ml. By duration of action insulins are divided into 4 main groups (Table 7.4):
Table 7.4. Pharmacokinetics of drugs and insulin analogues
 1.
Ultra-short-acting (lispro, aspart).
1.
Ultra-short-acting (lispro, aspart).
2. Short-acting (simple human insulin).
3. Medium-acting (neutral protamine Hagedorn insulins).
4. Long-acting (glargine, detemir).
5. Mixtures of insulins of varying duration of action (Novomix-30, Humulin-MZ, Humalog-Mix-25).
Drugs ultra-short action[lispro (Humalog), aspart (Novorapid)] are insulin analogues. Their advantages are the rapid development of the hypoglycemic effect after injection (after 15 minutes), which allows injection immediately before meals or even immediately after meals, as well as a short duration of action (less than 3 hours), which reduces the risk of hypoglycemia. Drugs short acting(simple insulin, regular insulin) are a solution containing insulin at a concentration of 100 U/ml. An injection of simple insulin is given 30 minutes before meals; The duration of action is about 4-6 hours. Ultra-short and short-acting drugs can be administered subcutaneously, intramuscularly and intravenously.
Among the drugs average duration of action The most commonly used drugs are neutral protamine Hagedorn (NPH). NPH is a protein that non-covalently adsorbs insulin, slowing its absorption from the subcutaneous depot. The effective duration of action of NPH insulins is usually about 12 hours; they are administered only subcutaneously. NPH insulin is a suspension, and therefore, unlike simple insulin, it is cloudy in the vial, and when left standing for a long time, a suspension is formed, which must be thoroughly mixed before injection. NPH insulins, unlike other long-acting drugs, can be mixed in any ratio with short-acting insulin (simple insulin), and the pharmacokinetics of the components of the mixture will not change, since NPH will not bind additional amounts of simple insulin (Fig. 7.5). In addition, protamine is used to prepare standard mixtures of insulin analogues (Novomix-30, Humalog-Mix-25).
Among long-acting drugs, insulin analogues are currently actively used. glargine(Lantus) and detemir(Levemir). A favorable feature of the pharmacokinetics of these drugs is that, unlike NPH insulins, they provide a more uniform and prolonged supply of the drug from the subcutaneous depot. In this regard, glargine can be prescribed only once a day, practically regardless of the time of day.
 Rice. 7.5. Pharmacokinetics of various insulin preparations:
Rice. 7.5. Pharmacokinetics of various insulin preparations:
a) monocomponent; b) standard insulin mixtures
In addition to monocomponent insulin preparations, they are widely used in clinical practice. standard mixtures. As a rule, we are talking about mixtures of short-acting or ultra-short-acting insulin with intermediate-acting insulin. For example, the drug “Humulin-MZ” contains 30% simple insulin and 70% NPH insulin in one bottle; the drug "Novomix-30" contains 30% insulin aspart and 70% crystalline protamine suspension of insulin aspart; the drug "Humalog-Mix-25" contains 25% insulin lispro and 75% protamine suspension of insulin lispro. Advantage
standard insulin mixtures are the replacement of two injections with one and somewhat greater accuracy in the dosage of the components of the mixture; The disadvantage is the impossibility of individual dosing of individual components of the mixture. This determines the preference for using standard insulin mixtures for the treatment of T2DM or the so-called traditional insulin therapy(prescribing fixed doses of insulin), whereas for intensive insulin therapy(flexible dose selection depending on glycemic indicators and the amount of carbohydrates in food) the use of monocomponent drugs is preferable.
The key to successful insulin therapy is strict adherence to injection techniques. There are several ways to administer insulin. The simplest and most reliable method is injection using insulin. syringe. A more convenient way to administer insulin is by injection using syringe pens, which is a combined device containing an insulin reservoir (cartridge), a dosing system and a needle with an injector.
For maintenance therapy (when we are not talking about severe decompensation of diabetes or critical conditions), insulin is administered subcutaneously. Injections of short-acting insulin are recommended to be made into the subcutaneous fatty tissue of the abdomen, long-acting insulin - into the tissue of the thigh or shoulder (Fig. 7.6 a). Injections are made deep into the subcutaneous tissue through widely compressed skin at an angle of 45° (Fig. 7.6 b). The patient should be advised to change insulin injection sites within the same area daily to prevent the development of lipodystrophies.
TO factors affecting the rate of insulin absorption from the subcutaneous depot, the dose of insulin should be taken into account (increasing the dose increases the duration of absorption), the injection site (absorption is faster from abdominal tissue), and the ambient temperature (warming and massaging the injection site accelerates absorption).
A more complex method of administration, which nevertheless allows achieving good treatment results in many patients, is the use of insulin dispenser, or systems for continuous subcutaneous insulin administration. The dispenser is a portable device consisting of a computer that sets the insulin supply mode, as well as an insulin supply system carried out through a catheter and a miniature needle into the subcutaneous
 Rice. 7.6. Insulin injections: a) typical injection sites; b) position of the insulin syringe needle during injection
Rice. 7.6. Insulin injections: a) typical injection sites; b) position of the insulin syringe needle during injection
fatty tissue. Using a dispenser, a continuous basal injection of short-acting or ultra-short-acting insulin is carried out (at a rate of about 0.5-1 U/hour), and before eating, depending on the carbohydrate content and glycemic level, the patient administers the required bolus dose of the same short-acting insulin. The advantage of insulin therapy using a dispenser is the administration of short-acting (or even ultra-short) insulin alone, which in itself is somewhat more physiological, since the absorption of long-acting insulin preparations is subject to large fluctuations; in this regard, continuous administration of short-acting insulin appears to be a more manageable process. The disadvantage of insulin therapy using a dispenser is the need to constantly wear the device, as well as the long-term presence of the injection needle in the subcutaneous tissue, which requires periodic monitoring of the insulin supply process. Insulin therapy using a dispenser is primarily indicated for patients with type 1 diabetes who are ready to master the technique of its management. Particularly in this regard, attention should be paid to patients with a pronounced “dawning” phenomenon, as well as to pregnant and planning pregnancy patients with T1DM and patients
ents with a disordered lifestyle (the possibility of a more flexible diet).
7.5. TYPE 1 DIABETES
CD-1 - organ-specific autoimmune a disease leading to the destruction of insulin-producing β-cells of the islets of the pancreas, manifested by an absolute deficiency of insulin. In some cases, patients with overt T1DM lack markers of autoimmune damage to β-cells (idiopathic DM-1).
Etiology
DM-1 is a disease with a hereditary predisposition, but its contribution to the development of the disease is small (determines its development by approximately 1/3). The concordance rate for T1DM in identical twins is only 36%. The probability of developing T1D in a child with a sick mother is 1-2%, for a father - 3-6%, for a brother or sister - 6%. One or more humoral markers of autoimmune β-cell damage, which include antibodies to pancreatic islets, antibodies to glutamate decarboxylase (GAD65) and antibodies to tyrosine phosphatase (IA-2 and ΙΑ-2β), are detected in 85-90% of patients . Nevertheless, the main role in the destruction of β-cells is given to cellular immunity factors. T1DM is associated with such HLA haplotypes as DQA And DQB while only alleles HLA-DR/DQ may be predisposing to the development of the disease, while others are protective. With an increased frequency, DM-1 is combined with other autoimmune endocrine (autoimmune thyroiditis, Addison's disease) and non-endocrine diseases, such as alopecia, vitiligo, Crohn's disease, rheumatic diseases (Table 7.5).
Pathogenesis
DM-1 manifests itself when 80-90% of β-cells are destroyed by an autoimmune process. The speed and intensity of this process can vary significantly. Most often when typical course diseases in children and young people, this process proceeds quite quickly, followed by a rapid manifestation of the disease, in which only a few weeks can pass from the appearance of the first clinical symptoms to the development of ketoacidosis (up to ketoacidotic coma).
Table 7.5. Diabetes mellitus type 1
 Continuation of the table. 7.5
Continuation of the table. 7.5
 In other, much rarer cases, usually in adults over 40 years of age, the disease may be latent. (latent autoimmune diabetes of adults - LADA), At the same time, at the onset of the disease, such patients are often diagnosed with diabetes mellitus-2, and for several years, compensation for diabetes can be achieved by prescribing sulfonylurea drugs. But later, usually after 3 years, signs of absolute insulin deficiency appear (weight loss, ketonuria, severe hyperglycemia, despite taking tableted hypoglycemic drugs).
In other, much rarer cases, usually in adults over 40 years of age, the disease may be latent. (latent autoimmune diabetes of adults - LADA), At the same time, at the onset of the disease, such patients are often diagnosed with diabetes mellitus-2, and for several years, compensation for diabetes can be achieved by prescribing sulfonylurea drugs. But later, usually after 3 years, signs of absolute insulin deficiency appear (weight loss, ketonuria, severe hyperglycemia, despite taking tableted hypoglycemic drugs).
The pathogenesis of T1DM, as indicated, is based on absolute insulin deficiency. The inability of glucose to enter insulin-dependent tissues (fat and muscle) leads to energy deficiency, resulting in intensified lipolysis and proteolysis, which are associated with weight loss. An increase in glycemic levels causes hyperosmolarity, which is accompanied by osmotic diuresis and severe dehydration. Under conditions of insulin deficiency and energy deficiency, the production of contrainsular hormones (glucagon, cortisol, growth hormone) is disinhibited, which, despite increasing glycemia, causes stimulation of gluconeogenesis. Increased lipolysis in adipose tissue leads to a significant increase in the concentration of free fatty acids. With insulin deficiency, the liposynthetic capacity of the liver is suppressed, and free
fatty acids begin to be included in ketogenesis. The accumulation of ketone bodies leads to the development of diabetic ketosis, and subsequently ketoacidosis. With a progressive increase in dehydration and acidosis, a coma develops (see section 7.7.1), which, in the absence of insulin therapy and rehydration, inevitably ends in death.
Epidemiology
T1DM accounts for about 1.5-2% of all cases of diabetes, and this relative figure will further decrease due to the rapid increase in the incidence of T2DM. The lifetime risk of developing T1DM in a Caucasian person is about 0.4%. The incidence of type 1 diabetes is increasing by 3% per year: by 1.5% due to new cases and by another 1.5% due to an increase in the life expectancy of patients. The prevalence of T1DM varies depending on the ethnic composition of the population. As of 2000, it was 0.02% in Africa, 0.1% in South Asia and South and Central America, and 0.2% in Europe and North America. The incidence of DM-1 is highest in Finland and Sweden (30-35 cases per 100 thousand population per year), and lowest in Japan, China and Korea (0.5-2.0 cases, respectively). The peak age for the manifestation of T1DM corresponds to approximately 10-13 years. In the vast majority of cases, T1DM manifests itself before the age of 40.
Clinical manifestations
IN typical cases, Especially in children and young people, T1DM debuts with a vivid clinical picture that develops over several months or even weeks. The manifestation of T1DM can be triggered by infectious and other concomitant diseases. Characteristic symptoms common to all types of diabetes, associated with hyperglycemia: polydipsia, polyuria, skin itching, but with type 1 diabetes they are very pronounced. So, throughout the day, patients can drink and excrete up to 5-10 liters of fluid. Specific for type 1 diabetes, the symptom, which is caused by an absolute deficiency of insulin, is weight loss reaching 10-15 kg over 1-2 months. Characterized by severe general and muscle weakness, decreased performance, and drowsiness. At the onset of the disease, some patients may experience an increase in appetite, which gives way to anorexia as ketoacidosis develops. The latter is characterized by the appearance of an acetone odor (or fruity odor) from the mouth, nausea
notes, vomiting, often abdominal pain (pseudoperitonitis), severe dehydration and ends in the development of a coma (see section 7.7.1). In some cases, the first manifestation of T1DM in children is a progressive impairment of consciousness up to coma due to concomitant diseases, usually infectious or acute surgical pathology.
In relatively rare cases of the development of T1DM in people over 35-40 years of age (latent autoimmune diabetes of adults) the disease may not manifest itself so clearly (moderate polydipsia and polyuria, no loss of body weight) and may even be detected by chance during routine determination of glycemic levels. In these cases, the patient is often initially diagnosed with diabetes mellitus-2 and is prescribed tableted hypoglycemic drugs (TGDs), which provide acceptable compensation for diabetes for some time. However, over several years (often within a year), the patient develops symptoms caused by an increasing absolute deficiency of insulin: weight loss, inability to maintain normal glycemia against the background of TSP, ketosis, ketoacidosis.
Diagnostics
Considering that DM-1 has a clear clinical picture and is also a relatively rare disease, screening determination of glycemic levels for the purpose of diagnosing DM-1 is not indicated. The likelihood of developing the disease in the patients' immediate relatives is low, which, together with the lack of effective methods for primary prevention of T1DM, determines the inappropriateness of studying immunogenetic markers of the disease in them. Diagnosis of T1DM in the vast majority of cases is based on the detection of significant hyperglycemia in patients with severe clinical manifestations of absolute insulin deficiency. OGTT for the purpose of diagnosing T1DM has to be performed very rarely.
Differential diagnosis
In doubtful cases (detection of moderate hyperglycemia in the absence of obvious clinical manifestations, manifestation at a relatively advanced age), as well as for the purpose of differential diagnosis with other types of diabetes, level determination is used C-peptide(basal and 2 hours after meals). In indirect diagnostic value in doubtful cases, the definition may have immunological markers CD-1 - antibodies to islets
PZH, to glutamate decarboxylase (GAD65) and tyrosine phosphatase (IA-2 and IA-2β). Differential diagnosis of DM-1 and DM-2 is presented in table. 7.6.
Table 7.6. Differential diagnosis and differences between DM-1 and DM-2
 Treatment
Treatment
Treatment of any type of diabetes is based on three main principles: glucose-lowering therapy (for diabetes-1 - insulin therapy), diet and patient education. Insulin therapy with DM-1 wears substitutive nature and its goal is to maximally imitate the physiological production of the hormone in order to achieve the accepted compensation criteria (Table 7.3). Closest to physiological insulin secretion intensive insulin therapy. The need for insulin corresponding to its basal secretion is provided by two injections of intermediate-acting insulin (morning and evening) or one injection of long-acting insulin (glargine). Total dose of basal insulin
The amount should not exceed half of the total daily requirement for the drug. Food or bolus insulin secretion is replaced by injections of short-acting or ultra-short-acting insulin before each meal, and its dose is calculated based on the amount of carbohydrates expected to be taken during the upcoming meal and the existing level of glycemia, determined by the patient using a glucometer before each insulin injection (Fig. 7.7 ).
Approximate intensive insulin therapy regimen, which will change almost every day, can be represented as follows. It is assumed that the daily need for insulin is about 0.5-0.7 units per 1 kg of body weight (for a patient weighing 70 kg, about 35-50 units). About 1/3 - 1/2 of this dose will be long-acting insulin (20-25 U), 1/2 - 2/3 of short- or ultra-short-acting insulin. The dose of NPH insulin is divided into 2 injections: in the morning 2/3 of its dose (12 units), in the evening - 1/3 (8-10 units).
Purpose first stage selection of insulin therapy is to normalize fasting glucose levels. The evening dose of NPH insulin is usually administered at 22-23 hours, the morning dose along with an injection of short-acting insulin before breakfast. When selecting an evening dose of NPH insulin, it is necessary to keep in mind the possibility of developing a number of
 Rice. 7.7. Intensive insulin therapy regimen
Rice. 7.7. Intensive insulin therapy regimen
quite typical phenomena. The cause of morning hyperglycemia may be an insufficient dose of long-acting insulin, since by the morning the need for insulin increases significantly (the “dawn” phenomenon). In addition to insufficient dose, morning hyperglycemia can be caused by its excess - Somogyi phenomenon(Somogyi), post-hypoglycemic hyperglycemia. This phenomenon is explained by the fact that the maximum sensitivity of tissues to insulin is observed between 2 and 4 am. It is at this time that the level of the main contrainsular hormones (cortisol, growth hormone, etc.) is normally the lowest. If the evening dose of long-acting insulin is excessive, then at this time hypoglycemia. Clinically, it can manifest itself as poor sleep with nightmares, unconscious actions in sleep, morning headaches and fatigue. The development of hypoglycemia at this time causes a significant compensatory release of glucagon and other contrainsular hormones, followed by hyperglycemia in the morning. If in this situation the dose of long-acting insulin administered in the evening is not reduced, but increased, nocturnal hypoglycemia and morning hyperglycemia will worsen, which can ultimately lead to chronic insulin overdose syndrome (Somogyi syndrome), which is a combination of obesity with chronic decompensation of diabetes, frequent hypoglycemia and progressive late complications. To diagnose the Somogyi phenomenon, it is necessary to study the glycemic level at about 3 a.m., which is an integral component of the selection of insulin therapy. If a decrease in the evening dose of NPH to a safe nocturnal hypoglycemia is accompanied by hyperglycemia in the morning (dawn phenomenon), the patient should be advised to rise earlier (6-7 am), while insulin administered at night continues to maintain normal glycemic levels.
A second injection of NPH insulin is usually given before breakfast along with the morning injection of short-acting (ultra-short-acting) insulin. In this case, the dose is selected primarily based on glycemic levels before the main daily meals (lunch, dinner); in addition, it can be limited by the development of hypoglycemia in the intervals between meals, for example at noon, between breakfast and lunch.
Whole dose of insulin long-acting(glargine) is administered once a day, and it does not matter at what time. Kinetics
insulin glargine and detemir are more favorable in terms of the risk of developing hypoglycemia, including nighttime ones.
The dose of short-acting or ultra-short-acting insulin, even on the first day of insulin prescription for the patient, will depend on the amount of carbohydrates consumed (bread units) and the level of glycemia before injection. Conventionally, based on the normal daily rhythm of insulin secretion, about 1/4 of the dose of short-acting insulin (6-8 units) is allocated for dinner, the remaining dose is divided approximately equally into breakfast and lunch (10-12 units). The higher the initial glycemic level, the less it will decrease per unit of insulin administered. A short-acting insulin injection is given 30 minutes before a meal, an ultra-short-acting insulin injection immediately before a meal, or even immediately after a meal. The adequacy of the dose of short-acting insulin is assessed by glycemic indicators 2 hours after meals and before the next meal.
To calculate the dose of insulin during intensive insulin therapy, it is sufficient to calculate the number of XE based only on the carbohydrate component. In this case, not all carbohydrate-containing products are taken into account, but only the so-called countable ones. The latter include potatoes, grain products, fruits, liquid dairy and sweet products. Products containing indigestible carbohydrates (most vegetables) are not taken into account. Special exchange tables have been developed with the help of which, by expressing the amount of carbohydrates in XE, you can calculate the required dose of insulin. One XE corresponds to 10-12 g of carbohydrates (Table 10.7).
After eating a meal containing 1 XE, the glycemic level increases by 1.6-2.2 mmol/l, i.e. approximately as much as the glucose level decreases when 1 unit of insulin is administered. In other words, for each XE contained in the food you plan to eat, you need to administer about 1 unit of insulin in advance (depending on the time of day). In addition, it is necessary to take into account the results of self-monitoring of glycemic levels, which is performed before each injection, and the time of day (about 2 U of insulin per 1 XE in the morning and at lunch, 1 U per 1 XE at dinner). So, if hyperglycemia is detected, the dose of insulin, calculated in accordance with the upcoming meal (based on the number of XE), needs to be increased, and vice versa, if hypoglycemia is detected, less insulin is administered.
Table 7.7. Equivalent replacement of products making up 1 XE
 For example, if a patient has a glycemic level of 7 mmol/l 30 minutes before a planned dinner containing 5 XE, he needs to inject 1 unit of insulin so that the glycemia decreases to a normal level: from 7 mmol/l to approximately 5 mmol/ l. In addition, 5 units of insulin must be administered to cover 5 XE. Thus, in this case, the patient will inject 6 units of short-acting or ultra-short-acting insulin.
For example, if a patient has a glycemic level of 7 mmol/l 30 minutes before a planned dinner containing 5 XE, he needs to inject 1 unit of insulin so that the glycemia decreases to a normal level: from 7 mmol/l to approximately 5 mmol/ l. In addition, 5 units of insulin must be administered to cover 5 XE. Thus, in this case, the patient will inject 6 units of short-acting or ultra-short-acting insulin.
After the manifestation of T1DM and the initiation of insulin therapy for a sufficiently long time, the need for insulin may be small and be less than 0.3-0.4 U/kg. This period is referred to as the remission phase, or "Honeymoon". After a period of hyperglycemia and ketoacidosis, which suppress insulin secretion by the 10-15% of remaining β-cells, compensation of hormonal-metabolic disorders by administration of insulin restores the function of these cells, which then take over providing the body with insulin at a minimum level. This period can last from several weeks to several years, but eventually, due to autoimmune destruction of the remaining β-cells, the “honeymoon” ends.
Diet for T1DM in trained patients who have the skills of self-control and selection of insulin dosage, it can be liberalized, i.e. approaching free. If the patient is not overweight or underweight, the diet should be
isocaloric. The main component of food for T1DM is carbohydrates, which should account for about 65% of daily calories. Preference should be given to products containing complex, slowly absorbed carbohydrates, as well as products rich in dietary fiber. Products containing easily digestible carbohydrates (flour, sweets) should be avoided. The proportion of proteins should be reduced to 10-35%, which helps reduce the risk of developing microangiopathy, and the proportion of fats should be reduced to 25-35%, while limiting fats should account for up to 7% of calories, which reduces the risk of developing atherosclerosis. In addition, it is necessary to avoid drinking alcoholic beverages, especially strong ones.
An integral component of working with a patient with T1DM and the key to effective compensation is patient education. Throughout his life, the patient must independently change the dose of insulin every day, depending on numerous factors. Obviously, this requires mastery of certain skills that need to be taught to the patient. “Patient School with DM-1” is organized in endocrinology hospitals or on an outpatient basis and consists of 5-7 structured sessions in which a doctor or specially trained nurse interactively, using various visual aids, teaches patients the principles self-control.
Forecast
In the absence of insulin therapy, a patient with type 1 diabetes inevitably dies from ketoacidotic coma. With inadequate insulin therapy, against the background of which the criteria for compensation of diabetes are not achieved and the patient is in a state of chronic hyperglycemia (Table 7.3), late complications begin to develop and progress (clause 7.8). In T1DM, the manifestations of diabetic microangiopathy (nephropathy and retinopathy) and neuropathy (diabetic foot syndrome) are of greatest clinical importance in this regard. Macroangiopathy in type 1 diabetes comes to the fore relatively rarely.
7.6. TYPE 2 DIABETES MELLITUS
Diabetes mellitus type 2- a chronic disease manifested by impaired carbohydrate metabolism with the development of hyperglycemia due to insulin resistance and secretory dysfunction of β-cells,
as well as lipid metabolism with the development of atherosclerosis. Since the main cause of death and disability in patients is complications of systemic atherosclerosis, T2DM is sometimes called cardiovascular disease.
Table 7.8. Diabetes mellitus type 2
 Etiology
Etiology
T2DM is a multifactorial disease with a hereditary predisposition. Concordance for T2DM in identical twins reaches 80% or more. Most patients with T2DM indicate the presence of T2DM in their immediate family; If one of the parents has T2DM, the probability of its development in a descendant throughout life is 40%. No single gene, the polymorphism of which determines predisposition to T2DM, has been found. Environmental factors, primarily lifestyle features, play a great role in the realization of a hereditary predisposition to T2DM. Risk factors for developing T2DM are:
Obesity, especially visceral (see paragraph 11.2);
Ethnicity (especially when changing a traditional lifestyle to a Western one);
Sedentary lifestyle;
Dietary features (high consumption of refined carbohydrates and low fiber content);
Arterial hypertension.
Pathogenesis
Pathogenetically, T2DM is a heterogeneous group of metabolic disorders, which is what determines its significant clinical heterogeneity. Its pathogenesis is based on insulin resistance (a decrease in insulin-mediated glucose utilization by tissues), which occurs against the background of secretory dysfunction of β-cells. Thus, there is an imbalance in insulin sensitivity and insulin secretion. Secretory dysfunctionβ -cells consists of slowing down the “early” secretory release of insulin in response to an increase in blood glucose levels. In this case, the 1st (fast) phase of secretion, which consists of emptying vesicles with accumulated insulin, is virtually absent; The 2nd (slow) phase of secretion occurs in response to stabilizing hyperglycemia constantly, in a tonic mode, and, despite excess insulin secretion, the level of glycemia against the background of insulin resistance does not normalize (Fig. 7.8).
The consequence of hyperinsulinemia is a decrease in the sensitivity and number of insulin receptors, as well as suppression
post-receptor mechanisms mediating the effects of insulin (insulin resistance). The content of the main glucose transporter in muscle and fat cells (GLUT-4) is reduced by 40% in individuals with visceral obesity and by 80% in individuals with type 2 diabetes. Due to insulin resistance of hepatocytes and portal hyperinsulinemia occurs overproduction of glucose by the liver, and fasting hyperglycemia develops, which is detected in most patients with T2DM, including in the early stages of the disease.
Hyperglycemia itself adversely affects the nature and level of secretory activity of β-cells (glucotoxicity). Long-term, over many years and decades, existing hyperglycemia eventually leads to depletion of β-cell insulin production and the patient may experience some symptoms insulin deficiency- weight loss, ketosis with concomitant infectious diseases. However, residual insulin production, which is sufficient to prevent ketoacidosis, is almost always preserved in T2DM.
Epidemiology
T2DM determines the epidemiology of diabetes as a whole, since it accounts for about 98% of cases of this disease. The prevalence of T2DM varies among countries and ethnic groups. In European
 Rice. 7.8. Secretory dysfunction of β-cells in type 2 diabetes mellitus (loss of the 1st fast phase of insulin secretion)
Rice. 7.8. Secretory dysfunction of β-cells in type 2 diabetes mellitus (loss of the 1st fast phase of insulin secretion)
countries, the USA and the Russian Federation, it makes up about 5-6% of the population. The incidence of T2DM increases with age: among adults, the prevalence of T2DM is 10%, and among people over 65 years of age it reaches 20%. The incidence of T2DM is 2.5 times higher among Native Americans and Hawaiians; among the Pima Indians (Arizona) it reaches 50%. Among the rural populations of India, China, Chile and African countries who lead a traditional lifestyle, the prevalence of T2DM is very low (less than 1%). On the other hand, among immigrants to Western industrial countries it reaches a significant level. Thus, among immigrants from India and China living in the USA and Great Britain, the prevalence of T2DM reaches 12-15%.
WHO predicts an increase in the number of people with diabetes in the world by 122% over the next 20 years (from 135 to 300 million). This is due both to the progressive aging of the population and to the spread and worsening of an urbanized lifestyle. In recent years, there has been a significant “rejuvenation” of T2DM and an increase in its incidence among children.
Clinical manifestations
In most cases, there are no pronounced clinical manifestations, and the diagnosis is established by routine determination of glycemic levels. The disease usually manifests itself over the age of 40 years, while the vast majority of patients have obesity and other components of the metabolic syndrome (see section 11.2). Patients do not complain about decreased performance if there are no other reasons for this. Complaints of thirst and polyuria rarely reach significant severity. Quite often, patients are bothered by skin and vaginal itching, and therefore they turn to dermatologists and gynecologists. Since many years (on average about 7 years) often pass from the actual manifestation of T2DM to diagnosis, in many patients the clinical picture is dominated by symptoms and manifestations of late complications of diabetes. Moreover, the first visit of a patient with T2DM to medical care very often occurs due to late complications. Thus, patients can be hospitalized in surgical hospitals with ulcerative lesions of the legs (diabetic foot syndrome), contact ophthalmologists due to progressive vision loss (diabetic retinopathy), be hospitalized with heart attacks, stroke
tami, obliterating lesions of the vessels of the legs in institutions where hyperglycemia is first detected.
Diagnostics
Diagnostic criteria, common for all types of diabetes, are presented in paragraph 7.3. The diagnosis of DM-2 in the vast majority of cases is based on the identification of hyperglycemia in individuals with typical clinical signs of DM-2 (obesity, age over 40-45 years, positive family history of DM-2, other components of the metabolic syndrome), in the absence of clinical and laboratory signs absolute insulin deficiency (pronounced weight loss, ketosis). The combination of the high prevalence of T2DM, its characteristic long-term asymptomatic course and the possibility of preventing its severe complications with early diagnosis predetermine the need screening, those. conducting an examination to exclude T2DM among persons without any symptoms of the disease. The main test, as indicated, is the determination fasting blood glucose level. It is indicated in the following situations:
1. In all people over 45 years of age, especially with excess body weight (BMI more than 25 kg/m2) at intervals of every 3 years.
2. At a younger age in the presence of excess body weight (BMI more than 25 kg/m2) and additional risk factors, which include:
Sedentary lifestyle;
CD-2 in close relatives;
Belonging to nationalities at high risk of developing T2DM (African Americans, Hispanics, Native Americans, etc.);
Women who gave birth to a child weighing more than 4 kg and/or with a history of gestational diabetes;
Arterial hypertension (≥ 140/90 mm Hg);
HDL level > 0.9 mmol/l and/or triglycerides > 2.8 mmol/l;
Polycystic ovary syndrome;
NTG and NGNT;
Cardiovascular diseases.
A significant increase in the incidence of T2DM among children dictates the need for screening determination of glycemic levels among children and adolescents(starting from 10 years with an interval of 2 years or with the beginning
puberty, if it occurred at an earlier age), belonging to high-risk groups, which include children with excess body weight(BMI and/or weight > 85th percentile for age or weight greater than 120% of ideal weight) in combination with any two of the following additional risk factors:
CD-2 among first- or second-degree relatives;
Belonging to high-risk nationalities;
Clinical manifestations associated with insulin resistance (acanthosis nigricans, arterial hypertension, dyslipidemia);
Diabetes, including gestational diabetes, in the mother.
Differential diagnosis
The differential diagnosis of DM-2 and DM-1 is of greatest clinical importance, the principles of which are described in paragraph 7.5 (Table 7.6). As indicated, in most cases it is based on clinical data. In cases where establishing the type of diabetes is difficult, or there is a suspicion of some rare variant of diabetes, including within the framework of hereditary syndromes, the most important practical question that needs to be answered is whether the patient needs insulin therapy.
Treatment
The main components of treatment for DM2 are: diet therapy, increased physical activity, hypoglycemic therapy, prevention and treatment of late complications of DM. Since most patients with T2DM are obese, the diet should be aimed at weight loss (hypocaloric) and the prevention of late complications, primarily macroangiopathy (atherosclerosis). Hypocaloric diet necessary for all patients with excess body weight (BMI 25-29 kg/m2) or obesity (BMI > 30 kg/m2). In most cases, it should be recommended to reduce the daily calorie intake to 1000-1200 kcal for women and to 1200-1600 kcal for men. The recommended ratio of the main food components for DM-2 is similar to that for DM-1 (carbohydrates - 65%, proteins 10-35%, fats up to 25-35%). Use alcohol must be limited due to the fact that it is a significant source of additional calories; in addition, alcohol intake during therapy
Ingestion of sulfonylureas and insulin can provoke the development of hypoglycemia (see section 7.7.3).
Recommendations for increasing physical activity must be individualized. At the beginning, aerobic exercise (walking, swimming) of moderate intensity lasting 30-45 minutes 3-5 times a day (about 150 minutes per week) is recommended. In the future, a gradual increase in physical activity is necessary, which significantly contributes to the reduction and normalization of body weight. In addition, physical activity helps reduce insulin resistance and has a hypoglycemic effect. The combination of diet therapy and increased physical activity without prescribing glucose-lowering drugs makes it possible to maintain diabetes compensation in accordance with established goals (Table 7.3) in approximately 5% of patients with T2DM.
Medicines for hypoglycemic therapy with T2DM can be divided into four main groups.
I. Drugs that help reduce insulin resistance (sensitizers). This group includes metformin and thiazolidinediones. Metformin is the only drug from the group currently in use biguanides. The main components of its mechanism of action are:
1. Suppression of gluconeogenesis in the liver (reduction of glucose production by the liver), which leads to a decrease in fasting blood glucose levels.
2. Reduced insulin resistance (increased glucose utilization by peripheral tissues, primarily muscles).
3. Activation of anaerobic glycolysis and reduction of glucose absorption in the small intestine.
Metformin is the drug of first choice for glucose-lowering therapy in patients with type 2 diabetes, obesity and fasting hyperglycemia. The starting dose is 500 mg at night or with dinner. Subsequently, the dose is gradually increased to 2-3 grams in 2-3 doses. Among the side effects, dyspepsia (diarrhea) is relatively common, which, as a rule, is transient and goes away on its own after 1-2 weeks of taking the drug. Since metformin does not have a stimulating effect on insulin production, hypoglycemia does not occur during monotherapy with this drug.
develop (its action will be designated as antihyperglycemic, and not hypoglycemic). Contraindications to the use of metformin are pregnancy, severe cardiac, hepatic, renal and other organ failure, as well as hypoxic conditions of other origins. An extremely rare complication that occurs when metformin is prescribed without taking into account the above contraindications is lactic acidosis, which is a consequence of hyperactivation of anaerobic glycolysis.
Thiazolidinediones(pioglitazone, rosiglitazone) are peroxisome proliferator-activated receptor γ (PPAR-γ) agonists. Thiazolidinediones activate the metabolism of glucose and lipids in muscle and adipose tissue, which leads to an increase in the activity of endogenous insulin, i.e. To eliminate insulin resistance (insulin sensitizers). The daily dose of pioglitazone is 15-30 mg/day, rosiglitazone - 4-8 mg (for 1-2 doses). The combination of thiazolidinediones with metformin is very effective. A contraindication to the use of thiazolidinediones is an increase (2.5 times or more) in the level of liver transaminases. In addition to hepatotoxicity, side effects of thiazolidinediones include fluid retention and edema, which more often develop when the drugs are combined with insulin.
II. Drugs affectingβ -cell and promote increased insulin secretion. This group includes sulfonylureas and glinides (prandial glycemic regulators), which are used primarily to normalize glycemic levels after meals. Main target sulfonylureas(PSM) are β-cells of pancreatic islets. PSMs bind to specific receptors on the β-cell membrane. This leads to the closure of ATP-dependent potassium channels and depolarization of the cell membrane, which in turn promotes the opening of calcium channels. The entry of calcium into β-cells leads to their degranulation and the release of insulin into the blood. In clinical practice, quite a lot of PSMs are used, which differ in the duration and severity of the glucose-lowering effect (Table 7.9).
Table 7.9. Sulfonylureas
 The main and fairly common side effect of PSM is hypoglycemia (see section 7.7.3). It can occur with an overdose of the drug, its accumulation (renal failure),
The main and fairly common side effect of PSM is hypoglycemia (see section 7.7.3). It can occur with an overdose of the drug, its accumulation (renal failure),
non-compliance with the diet (skipping meals, drinking alcohol) or regimen (significant physical activity, before which the dose of PSM was not reduced or carbohydrates were not taken).
To the group glinides(prandial glycemic regulators) include repaglinide(benzoic acid derivative; daily dose 0.5-16 mg/day) and nateglinide(D-phenylalanine derivative; daily dose 180-540 mg/day). Once administered, the drugs rapidly and reversibly interact with the sulfonylurea receptor on the β-cell, resulting in a short increase in insulin levels that mimics the first phase of normal insulin secretion. The drugs are taken 10-20 minutes before main meals, usually 3 times a day.
III. Drugs that reduce the absorption of glucose in the intestine.
This group includes acarbose and guar gum. The mechanism of action of acarbose is a reversible blockade of α-glycosidases in the small intestine, as a result of which the processes of sequential fermentation and absorption of carbohydrates are slowed down, the rate of resorption and entry of glucose into the liver is reduced, and the level of postprandial glycemia is reduced. The initial dose of acarbose is 50 mg 3 times a day, subsequently the dose can be increased to 100 mg 3 times a day; the drug is taken immediately before or during meals. The main side effect of acarbose is intestinal dyspepsia (diarrhea, flatulence), which is associated with the entry of unabsorbed carbohydrates into the colon. The glucose-lowering effect of acarbose is very moderate (Table 7.10).
In clinical practice, tableted hypoglycemic drugs are effectively combined with each other and with insulin drugs, since in most patients both fasting and postprandial hyperglycemia are simultaneously detected. There are numerous fixed combinations drugs in one tablet. Most often, metformin is combined with various PSMs in one tablet, as well as metformin with thiazolidinediones.
Table 7.10. Mechanism of action and potential effectiveness of tableted hypoglycemic drugs
 IV. Insulins and insulin analogues
IV. Insulins and insulin analogues
At a certain stage, up to 30-40% of patients with T2DM begin to receive insulin preparations. Indications for insulin therapy for type 2 diabetes are given at the beginning of section 7.4. The most common option for transferring patients with T2DM to insulin therapy is to prescribe long-acting insulin (NPH insulin, glargine or detemir) in combination with tablets of glucose-lowering drugs. In a situation where the fasting blood glucose level cannot be controlled by prescribing metformin or the latter is contraindicated, the patient is prescribed an evening (at night) insulin injection. If it is impossible to control both fasting and postprandial glycemia using tablet drugs, the patient is transferred to monoinsulin therapy. Typically, for T2DM, insulin therapy is carried out according to the so-called "traditional" scheme which involves prescribing fixed doses of long-acting and short-acting insulin. In this plan
Standard insulin mixtures containing short (ultra-short) and long-acting insulin in one bottle are convenient. The choice of traditional insulin therapy is determined by the fact that in case of T2DM, it is often prescribed to elderly patients, who are difficult to teach to independently change the insulin dose. In addition, intensive insulin therapy, the goal of which is to maintain compensation of carbohydrate metabolism at a level approaching normoglycemia, carries an increased risk of hypoglycemia. While mild hypoglycemia does not pose a serious risk in younger patients, it can have very adverse cardiovascular consequences in older patients with a lower threshold for experiencing hypoglycemia. Young patients with T2DM, as well as patients with promising opportunities for effective learning, may be prescribed an intensive version of insulin therapy.
Forecast
The main cause of disability and death in patients with T2DM are late complications (see section 7.8), most often diabetic macroangiopathy. The risk of developing certain late complications is determined by a complex of factors that are discussed in the relevant chapters. A universal risk factor for their development is chronic hyperglycemia. Thus, a decrease in HbA1c levels in patients with type 2 diabetes by 1% leads to a decrease in overall mortality by approximately 20%, by 2% and 3% - by approximately 40%, respectively.
7.7. ACUTE COMPLICATIONS OF DIABETES MELLITUS
7.7.1. Diabetic ketoacidosis
Diabetic ketoacidosis (DKA)- decompensation of DM-1, caused by an absolute deficiency of insulin, which, in the absence of timely treatment, ends in ketoacidotic coma (KC) and death.
Etiology
DKA is caused by an absolute deficiency of insulin. DKA of varying severity is determined in most patients at the time of manifestation of T1DM (10-20% of all cases of DKA).
In a patient with an established diagnosis of T1DM, DKA may develop when insulin administration is stopped, often by the patient himself (13% of DKA cases), against the background of concomitant diseases, primarily infectious, in the absence of an increase in the insulin dose
Table 7.11. Diabetic ketoacidosis
 Up to 20% of cases of DKA development in young patients with T1DM are associated with psychological problems and/or eating disorders (fear of weight gain, fear of hypoglycemia, teenage problems). A fairly common cause of DKA in a number of countries is
Up to 20% of cases of DKA development in young patients with T1DM are associated with psychological problems and/or eating disorders (fear of weight gain, fear of hypoglycemia, teenage problems). A fairly common cause of DKA in a number of countries is
withdrawal of insulin by the patient himself due to the high cost of drugs for some segments of the population (Table 7.11).
Pathogenesis
The pathogenesis of DKA is based on an absolute deficiency of insulin combined with an increase in the production of counter-insular hormones such as glucagon, catecholamines and cortisol. As a result, there is a significant increase in the production of glucose by the liver and a violation of its utilization by peripheral tissues, an increase in hyperglycemia and a violation of the osmolarity of the extracellular space. Insulin deficiency in combination with a relative excess of contrainsular hormones in DKA leads to the release of free fatty acids into the circulation (lipolysis) and their uncontrolled oxidation in the liver to ketone bodies (β-hydroxybutyrate, acetoacetate, acetone), resulting in the development of hyperketonemia, and subsequently metabolic acidosis. As a result of severe glycosuria, osmotic diuresis, dehydration, loss of sodium, potassium and other electrolytes develop (Fig. 7.9).
Epidemiology
The incidence of new cases of DKA is 5-8 per 1000 patients with type 1 diabetes per year and directly depends on the level of organization of medical care for patients with diabetes. There are approximately 100,000 hospitalizations for DKA each year in the United States, and with a per-patient cost of $13,000 per hospitalization, more than $1 billion per year is spent on inpatient care for DKA. In the Russian Federation in 2005, DKA was recorded in 4.31% of children, 4.75% of adolescents and 0.33% of adult patients with T1DM.
Clinical manifestations
The development of DKA, depending on the cause that caused it, can take from several weeks to a day. In most cases, DKA is preceded by symptoms of decompensated diabetes, but sometimes they may not have time to develop. Clinical symptoms of DKA include polyuria, polydipsia, weight loss, diffuse abdominal pain (“diabetic pseudoperitonitis”), dehydration, severe weakness, acetone odor from the breath (or fruity odor), and gradual clouding of consciousness. True coma with DKA has recently developed relatively rarely due to early diagnosis. Physical examination reveals signs of dehydration: decreased
 Rice. 7.9. Pathogenesis of ketoacidotic coma
Rice. 7.9. Pathogenesis of ketoacidotic coma
skin turgor and eyeball density, tachycardia, hypotension. In advanced cases, Kussmaul breathing develops. More than 25% of patients with DKA develop vomit, which may resemble coffee grounds in color.
Diagnostics
Based on clinical picture data, indications that the patient has type 1 diabetes, as well as laboratory test data. DKA is characterized by hyperglycemia (in some cases slight), ketonuria, metabolic acidosis, hyperosmolarity (Table 7.12).
Table 7.12. Laboratory diagnosis of acute complications of diabetes mellitus
 When examining patients with acute decompensation of diabetes, it is necessary to determine the level of glycemia, creatinine and urea, and electrolytes, on the basis of which the effective osmolarity is calculated. In addition, an assessment of the acid-base status is necessary. Effective Osmolarity(EO) is calculated using the following formula: 2 *. Normally, EO is 285 - 295 mOsm/l.
When examining patients with acute decompensation of diabetes, it is necessary to determine the level of glycemia, creatinine and urea, and electrolytes, on the basis of which the effective osmolarity is calculated. In addition, an assessment of the acid-base status is necessary. Effective Osmolarity(EO) is calculated using the following formula: 2 *. Normally, EO is 285 - 295 mOsm/l.
In most patients with DKA, leukocytosis, the severity of which is proportional to the level of ketone bodies in the blood. Level sodium, as a rule, it is reduced due to the osmotic outflow of fluid from the intracellular spaces to the extracellular spaces in response to hyperglycemia. Less commonly, sodium levels may be falsely reduced as a consequence of severe hyper-
triglyceridemia. Level potassium serum level may initially be increased due to its movement from the extracellular spaces.
Differential diagnosis
Other causes of loss of consciousness in patients with diabetes. Differential diagnosis with hyperosmolar coma, as a rule, does not cause difficulties (develops in elderly patients with T2DM) and does not have much clinical significance, because The treatment principles for both conditions are similar. If it is impossible to promptly determine the cause of loss of consciousness in a patient with diabetes, he is advised to administer glucose, because hypoglycemic conditions are much more common, and rapid positive dynamics against the background of glucose administration in itself makes it possible to find out the cause of loss of consciousness.
Treatment
Treatment of DKA involves rehydration, correction of hyperglycemia, electrolyte disorders, as well as treatment of diseases that have caused decompensation of diabetes. Treatment is most optimally carried out in the intensive care unit of a specialized medical institution. In adult patients without severe concomitant cardiac pathology, even at the prehospital stage as a first-priority measure to rehydration It is recommended to administer an isotonic solution (0.9% NaCl) at approximately a rate of one liter per hour (about 15-20 ml per kilogram of body weight per hour). Full compensation of fluid deficiency, which in DKA is 100-200 ml per kg of weight, should be achieved within the first day of treatment. With concomitant cardiac or renal failure, this period of time should be increased. For children, the recommended volume of isotonic solution for rehydration therapy is 10-20 ml per kg of body weight per hour, while in the first 4 hours it should not exceed 50 ml per kg of body weight. It is recommended to achieve complete rehydration after approximately 48 hours. After the level of glycemia decreases to approximately 14 mmol/l against the background of parallel insulin therapy, they switch to transfusion of a 10% glucose solution, which continues rehydration.
The concept of “small doses” is currently accepted insulin in the treatment of DKA. Only short-acting insulin is used. The most optimal use of intravenous insulin is
Lina. Intramuscular administration of insulin, which is less effective, is possible only with moderate severity of DKA, with stable hemodynamics and when intravenous therapy is not possible. In the latter case, injections are made into the rectus abdominis muscle, while an intramuscular injection needle is placed on the insulin syringe (for reliable intramuscular injection), and insulin is drawn from the vial into the syringe using this needle.
There are several options for intravenous insulin administration. Firstly, insulin can be injected “into the rubber band” of the infusion system, while the required amount of insulin is drawn into an insulin syringe, after which 1 ml of an isotonic solution is added to it. Until the glycemic level reaches 14 mmol/l, the patient is given 6-10 units of short-acting insulin every hour; further (in parallel with changing the rehydration solution from isotonic to 10% glucose) depending on the hourly determined glycemic indicators, the insulin dose is reduced to 4-8 units per hour. The recommended rate of reduction in glycemic levels should not exceed 5 mmol/l per hour. Another option for intravenous insulin therapy involves the use of a perfuser. To prepare a solution for the perfuser, proceed from the following ratio: 2 ml of a 20% solution of human albumin is added to 50 U of short-acting insulin, after which 50 mg of a 0.9% isotonic solution is added. If the intramuscular route of insulin administration is chosen, 20 units of short-acting insulin are initially administered, followed by 6 units hourly, and after the glycemic level reaches 14 mmol/l, the dose is reduced to 4 units per hour. After complete stabilization of hemodynamics and compensation of acid-base disorders, the patient is transferred to subcutaneous insulin injections.
As indicated, despite the significant potassium deficiency in the body (total loss 3-6 mmol/kg), with DKA its level before the start of insulin therapy may be slightly increased. However, it is recommended to start a transfusion of potassium chloride solution at the same time as starting insulin therapy if the plasma potassium level is less than 5.5 mmol/L. Successful correction of potassium deficiency occurs only against the background of normalization of pH. At low pH, the flow of potassium into the cell is significantly reduced; therefore, if possible, it is advisable to adapt the dose of potassium chloride transfused to a specific pH value (Table 7.13).
Table 7.13. Potassium deficiency correction scheme
 * The following data is used for calculations:
* The following data is used for calculations:
1 g KCl = 13.4 mmol; 1 mmol KCl = 0.075 g. In a 4% solution of KS1: in 100 ml - 4 g of KS1, in 25 ml - 1 g of KS1, in 10 ml 0.4 g of KS1.
Diabetes decompensation is often caused by infectious diseases(pyelonephritis, infected ulcer in diabetic foot syndrome, pneumonia, sinusitis, etc.). There is a rule according to which, in case of DKA, antibiotic therapy is prescribed to almost all patients with low-grade fever or fever, even in the absence of a visible source of infection, since an increase in body temperature is not typical for DKA.
Forecast
The mortality rate for DKA is 0.5-5%, with most cases due to late and unqualified medical care. Mortality is highest (up to 50%) among elderly patients.
7.7.2. Hyperosmolar coma
Hyperosmolar coma(GOC) is a rare acute complication of T2DM, developing as a result of severe dehydration and hyperglycemia in the absence of absolute insulin deficiency, accompanied by high mortality (Table 7.14).
Etiology
GOC usually develops in older patients with T2DM. Such patients are most often alone, live without care, neglect their condition and self-control, and take insufficient fluids. Often decompensation is caused by infections (diabetic foot syndrome, pneumonia, acute pyelonephritis), brain disorders
blood circulation and other conditions, as a result of which patients move poorly, do not take glucose-lowering drugs and fluids.
Table 7.14. Hyperosmolar coma (HOC)
 Pathogenesis
Pathogenesis
Increasing hyperglycemia and osmotic diuresis cause severe dehydration, which, for the above reasons, is not replenished from the outside. The result of hyperglycemia and dehydration is plasma hyperosmolarity. An integral component of the pathogenesis of GOC is a relative deficiency of insulin and an excess of contrainsular hormones; however, the residual insulin secretion that persists in T2DM is sufficient to suppress lipolysis and ketogenesis, as a result of which the development of ketoacidosis does not occur.
In some cases, moderate acidosis may be detected as a result of hyperlactatemia against the background of tissue hypoperfusion. With severe hyperglycemia, to maintain osmotic balance in the cerebrospinal fluid, the sodium content increases, coming from brain cells, where potassium enters in exchange. The transmembrane potential of nerve cells is disrupted. Progressive stupefaction develops in combination with convulsive syndrome (Fig. 7.10).
Epidemiology
GOCs account for 10-30% of acute hyperglycemic conditions in adults and elderly patients with T2DM. In approximately 2/3 of cases, GOC develops in individuals with previously undiagnosed diabetes.
Clinical manifestations
Features of the clinical picture of hyperosmolar coma are:
A set of signs and complications of dehydration and hypoperfusion: thirst, dry mucous membranes, tachycardia, arterial hypotension, nausea, weakness, shock;
Focal and generalized seizures;
Fever, nausea and vomiting (40-65% of cases);
Concomitant diseases and complications often include deep vein thrombosis, pneumonia, cerebrovascular accidents, and gastroparesis.
Diagnostics
Based on the clinical picture, the patient’s age and history of type 2 diabetes, severe hyperglycemia in the absence of ketonuria and ketoacidosis. Typical laboratory signs of GOC are presented in Table. 7.12.
 Rice. 7 .10.
Pathogenesis of hyperosmolar coma
Rice. 7 .10.
Pathogenesis of hyperosmolar coma
Differential diagnosis
Other acute conditions developing in patients with diabetes, most often with concomitant pathology, leading to severe decompensation of diabetes.
Treatment
Treatment and monitoring for GOC, with the exception of some features, do not differ from those described for ketoacidotic diabetic coma (section 7.7.1):
Larger volume of initial rehydration 1.5-2 liters per 1st hour; 1 l - in the 2nd and 3rd hours, then 500 ml/hour of isotonic sodium chloride solution;
The need for administration of potassium-containing solutions is, as a rule, greater than in ketoacidotic coma;
Insulin therapy is similar to that for CC, but the need for insulin is less and the glycemic level must be reduced no faster than 5 mmol/l per hour to avoid the development of cerebral edema;
Administration of a hypotonic solution (NaCl 0.45%) is best avoided (only with severe hypernatremia: > 155 mmol/l and/or effective osmolarity > 320 mOsm/l);
There is no need to administer bicarbonate (only in specialized intensive care units for acidosis with pH< 7,1).
Forecast
Mortality with GOC is high and ranges from 15-60%. The worst prognosis is in elderly patients with severe concomitant pathology, which is often the cause of decompensation of diabetes and the development of GOC.
7.7.3. Hypoglycemia
Hypoglycemia- decrease in serum glucose levels (<2,2- 2,8 ммоль/л), сопровождающее клинический синдром, характеризующийся признаками активации симпатической нервной системы и/или дисфункцией центральной нервной системы. Гипогликемия как лабораторный феномен не тождественен понятию «гипогликемическая симптоматика», поскольку лабораторные данные и клиническая картина не всегда совпадают.
Etiology
Overdose of insulin and its analogues, as well as sulfonylureas;
Insufficient food intake against the background of unchanged glucose-lowering therapy;
Drinking alcoholic beverages;
Physical activity against the background of constant glucose-lowering therapy and/or without additional intake of carbohydrates;
Development of late complications of diabetes (autonomous neuropathy with gastroparesis, renal failure) and a number of other diseases (adrenal insufficiency, hypothyroidism, liver failure, malignant tumors) with unchanged glucose-lowering therapy (continuation of taking and accumulation of TSP against the background of renal failure, maintaining the same dose of insulin);
Violation of insulin administration technique (intramuscular injection instead of subcutaneous);
Artificial hypoglycemia (deliberate overdose of glucose-lowering drugs by the patient himself);
Organic hyperinsulinism - insulinoma (see paragraph 10.3).
Pathogenesis
The pathogenesis of hypoglycemia is an imbalance between the entry of glucose into the blood, its utilization, the level of insulin and counter-insular hormones. Normally, at a glycemic level in the range of 4.2-4.7 mmol/l, the production and release of insulin from β-cells is suppressed. A decrease in glycemic levels below 3.9 mmol/l is accompanied by stimulation of the production of counter-insular hormones (glucagon, cortisol, growth hormone, adrenaline). Neuroglycopenic symptoms develop when the glycemic level decreases to less than 2.5-2.8 mmol/l. In case of overdose insulin and/or drugs sulfonylureas hypoglycemia develops due to the direct hypoglycemic effect of an exogenous or endogenous hormone. In the case of an overdose of sulfonylurea drugs, hypoglycemic symptoms can recur many times after the attack is stopped due to the fact that the duration of action of some drugs can reach a day or more. TSPs that do not have a stimulating effect on insulin production (metformin, thiazolidinediones) cannot cause hypoglycemia on their own, but when they are added to sulfonylureas or insulin, taking the latter in the same dose can cause hypoglycemia due to the accumulation of the hypoglycemic effect of combination therapy (Table 7.15).
Table 7.15. Hypoglycemia
 End of table. 7.15
End of table. 7.15
 Upon admission alcohol gluconeogenesis in the liver is suppressed, which is the most important factor counteracting hypoglycemia. Physical exercise promote insulin-independent utilization of glucose, due to which, against the background of unchanged glucose-lowering therapy and/or in the absence of additional carbohydrate intake, they can cause hypoglycemia.
Upon admission alcohol gluconeogenesis in the liver is suppressed, which is the most important factor counteracting hypoglycemia. Physical exercise promote insulin-independent utilization of glucose, due to which, against the background of unchanged glucose-lowering therapy and/or in the absence of additional carbohydrate intake, they can cause hypoglycemia.
Epidemiology
Mild, quickly reversible hypoglycemia in patients with type 1 diabetes receiving intensive insulin therapy can occur several times a week and is relatively harmless. For every patient on intensive insulin therapy, there is 1 case of severe hypoglycemia per year. In most cases, hypoglycemia develops at night. In type 2 diabetes, 20% of patients receiving insulin and 6% of patients receiving sulfonylureas develop at least one episode of severe hypoglycemia over 10 years.
Clinical manifestations
There are two main groups of symptoms: adrenergic, associated with activation of the sympathetic nervous system and the release of adrenaline by the adrenal glands, and neuroglycopenic, associated with dysfunction of the central nervous system against the background of a deficiency of its main energy substrate. TO adrenergic symptoms include: tachycardia, mydriasis; anxiety, aggressiveness; trembling, cold sweat, paresthesia; nausea, severe hunger, hypersalivation; diarrhea, excessive urination. TO neuroglycopenic symptoms include asthenia,
decreased concentration, headache, fear, confusion, disorientation, hallucinations; speech, visual, behavioral disorders, amnesia, impaired consciousness, convulsions, transient paralysis, coma. There may not be a clear relationship between the severity and sequence of symptoms as hypoglycemia becomes more severe. Only adrenergic or only neuroglycopenic symptoms may occur. In some cases, despite restoration of normoglycemia and ongoing therapy, patients may remain in a stuporous or even comatose state for several hours or even days. Prolonged hypoglycemia or its frequent episodes can lead to irreversible changes in the central nervous system (primarily in the cerebral cortex), the manifestations of which vary significantly from delirious and hallucinatory-paranoid episodes to typical epileptic seizures, the inevitable outcome of which is persistent dementia.
Hyperglycemia is subjectively tolerated by patients more easily than episodes of even mild hypoglycemia. Therefore, many patients, due to fear of hypoglycemia, consider it necessary to maintain glycemia at a relatively high level, which actually corresponds to decompensation of the disease. Overcoming this stereotype sometimes requires considerable effort from doctors and training staff.
Diagnostics
The clinical picture of hypoglycemia in a patient with diabetes in combination with laboratory (usually using a glucometer) detection of low blood glucose levels.
Differential diagnosis
Other causes leading to loss of consciousness. If the cause of loss of consciousness of a patient with diabetes is unknown and it is impossible to conduct a rapid analysis of the glycemic level, glucose administration is indicated. There is often a need to determine the cause of the development of frequent hypoglycemia in patients with diabetes. Most often they are the result of inadequate glucose-lowering therapy and the patient’s low level of knowledge about his disease. It should be remembered that a number of diseases (adrenal insufficiency, hypothyroidism, renal and liver failure), including malignant tumors, can lead to a reduction in the need for glucose-lowering therapy, up to its complete abolition (“disappeared diabetes”).
Treatment
To treat mild hypoglycemia, in which the patient is conscious and can help himself, it is usually sufficient to take food or liquid containing carbohydrates in the amount of 1-2 bread units (10-20 g of glucose). This amount is contained, for example, in 200 ml of sweet fruit juice. Drinks relieve hypoglycemia more effectively, since in liquid form glucose is absorbed much more quickly. If symptoms continue to worsen despite continued carbohydrate intake, intravenous glucose or intramuscular glucagon is necessary. Severe hypoglycemia that occurs with loss of consciousness is treated in a similar way. In this case, the patient is given about 50 ml 40% glucose solution intravenously. The administration of glucose must be continued until the attack is stopped and glycemia is normalized, although a larger dose - up to 100 ml or more, as a rule, is not required. Glucagon is administered (usually with a factory-prepared filled syringe) intramuscularly or subcutaneously. After a few minutes, the glycemic level returns to normal due to the induction of glycogenolysis by glucagon. However, this does not always happen: when the level of insulin in the blood is high, glucagon is ineffective. The half-life of glucagon is shorter than that of insulin. In alcoholism and liver disease, glycogen synthesis is impaired, and glucagon administration may be ineffective. A side effect of glucagon administration may be vomiting, creating the risk of aspiration. It is advisable for the patient’s relatives to master the technique of glucagon injection.
Forecast
Mild hypoglycemia in trained patients against the background of good compensation of the disease is safe. Frequent hypoglycemia is a sign of poor compensation of diabetes; in most cases, such patients have more or less severe hyperglycemia and a high level of glycated hemoglobin during the rest of the day. In elderly patients with late complications of diabetes, hypoglycemia can provoke vascular complications such as myocardial infarction, stroke, and retinal hemorrhage. Hypoglycemic coma lasting up to 30 minutes with adequate treatment and rapid return of consciousness, as a rule, does not have any complications or consequences.
7.8. LATE COMPLICATIONS OF DIABETES MELLITUS
Late complications develop in both types of diabetes. Clinically, there are five main late complications of diabetes: macroangiopathy, nephropathy, retinopathy, neuropathy and diabetic foot syndrome. The nonspecificity of late complications for certain types of diabetes is determined by the fact that their main pathogenetic link is chronic hyperglycemia. In this regard, at the time of manifestation of T1DM, late complications in patients almost never occur, developing over years and decades, depending on the effectiveness of the therapy. As a rule, the greatest clinical significance for T1DM is diabetic microangiopathy(nephropathy, retinopathy) and neuropathy (diabetic foot syndrome). With T2DM, on the contrary, late complications are often detected already at the time of diagnosis. Firstly, this is due to the fact that T2DM manifests itself long before the diagnosis is made. Secondly, atherosclerosis, clinically manifested by macroangiopathy, has many pathogenesis links in common with diabetes. In T2DM, the greatest clinical significance, as a rule, is acquired by diabetic macroangiopathy, which is detected in the vast majority of patients at the time of diagnosis. In each specific case, the set and severity of individual late complications vary from their paradoxical complete absence, despite the significant duration of the disease, to a combination of all possible options in a severe form.
Late complications are main cause of death patients with diabetes, and taking into account its prevalence, it is the most important medical and social health problem in most countries. Due to this main goal of treatment and observation of patients with diabetes is the prevention (primary, secondary, tertiary) of its late complications.
7.8.1. Diabetic macroangiopathy
Diabetic macroangiopathy- a collective concept that unites atherosclerotic lesions of large arteries in diabetes,
clinically manifested by coronary heart disease (CHD), obliterating atherosclerosis of the vessels of the brain, lower extremities, internal organs and arterial hypertension (Table 7.16).
Table 7.16. Diabetic macroangiopathy
 Etiology and pathogenesis
Etiology and pathogenesis
Probably similar to the etiology and pathogenesis of atherosclerosis in individuals without diabetes. Atherosclerotic plaques do not differ in microscopic structure in individuals with and without diabetes. However, in diabetes, additional risk factors may come to the fore, or diabetes may exacerbate known nonspecific factors. These for diabetes include:
1. Hyperglycemia. It is a risk factor for the development of atherosclerosis. An increase in HbA1c levels by 1% in patients with T2DM increases
There is a 15% risk of developing myocardial infarction. The mechanism of the atherogenic effect of hyperglycemia is not entirely clear; perhaps it is associated with glycosylation of the end products of LDL metabolism and collagen of the vascular wall.
2. Arterial hypertension(AG). In pathogenesis, great importance is attached to the renal component (diabetic nephropathy). Hypertension in type 2 diabetes is no less a significant risk factor for heart attack and stroke than hyperglycemia.
3. Dyslipidemia. Hyperinsulinemia, which is an integral component of insulin resistance in type 2 diabetes, causes a decrease in HDL levels, an increase in triglyceride levels and a decrease in density, i.e. increased atherogenicity of LDL.
4. Obesity, which most patients with T2DM suffer, is an independent risk factor for atherosclerosis, myocardial infarction and stroke (see section 11.2).
5. Insulin resistance. Hyperinsulinemia and high levels of insulin-proinsulin-like molecules increase the risk of developing atherosclerosis, which may be associated with endothelial dysfunction.
6. Blood coagulation disorder. In diabetes, an increase in the level of fibrinogen, platelet inhibitor activator and von Willebrand factor is determined, resulting in the formation of a prothrombotic state of the blood coagulation system.
7. Endothelial dysfunction, characterized by increased expression of plasminogen inhibitor activator and cell adhesion molecules.
8. Oxidative stress, leading to an increase in the concentration of oxidized LDL and F2-isoprostanes.
9. Systemic inflammation in which there is an increase in the expression of fibrinogen and C-reactive protein.
The most significant risk factors for the development of coronary artery disease in type 2 diabetes are increased LDL levels, decreased HDL levels, arterial hypertension, hyperglycemia and smoking. One of the differences between the atherosclerotic process in diabetes is its more widespread and distal nature of the occlusal lesion, those. The process often involves relatively smaller arteries, which complicates surgical treatment and worsens the prognosis.
Epidemiology
The risk of developing coronary heart disease in people with type 2 diabetes is 6 times higher than in people without diabetes, while it is the same for men and women. Arterial hypertension is detected in 20% of patients with type 1 diabetes and in 75% of patients with type 2 diabetes. In general, in patients with diabetes it occurs 2 times more often than in people without it. Obliterating atherosclerosis of peripheral vessels develops in 10% of patients with diabetes. Thromboembolism of cerebral vessels develops in 8% of patients with diabetes (2-4 times more often than in persons without diabetes).
Clinical manifestations
Basically they do not differ from those in persons without diabetes. In the clinical picture of T2DM, macrovascular complications (myocardial infarction, stroke, occlusive lesions of the vessels of the legs) often come to the fore, and it is with their development that hyperglycemia is often first detected in the patient. Perhaps due to concomitant autonomic neuropathy, up to 30% of myocardial infarctions in people with diabetes occur without a typical anginal attack (painless infarction).
Diagnostics
The principles for diagnosing complications of atherosclerosis (coronary heart disease, cerebrovascular accident, occlusive lesions of the arteries of the legs) do not differ from those for persons without diabetes. Measurement blood pressure(BP) should be carried out at every visit of a patient with diabetes to the doctor, and the determination of indicators lipid spectrum Blood tests (total cholesterol, triglycerides, LDL, HDL) for diabetes should be performed at least once a year.
Differential diagnosis
Other cardiovascular diseases, symptomatic arterial hypertension, secondary dyslipidemia.
Treatment
♦ Blood pressure control. The proper level of systolic blood pressure in diabetes is less than 130 mmHg, and diastolic blood pressure is 80 mmHg (Table 7.3). Most patients require multiple antihypertensive medications to achieve this goal. The drugs of choice for antihypertensive therapy for diabetes are ACE inhibitors and angiotensin receptor blockers, which, if necessary, are supplemented with thiazide diuretics. The drugs of choice for patients with diabetes who have suffered a myocardial infarction are β-blockers.
♦ Correction of dyslipidemia. Target levels of lipid spectrum indicators are presented in table. 7.3. The drugs of choice for lipid-lowering therapy are 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors (statins).
♦ Antiplatelet therapy. Aspirin therapy (75-100 mg/day) is indicated for patients with diabetes over 40 years of age with an increased risk of developing cardiovascular pathology (complicated family history, arterial hypertension, smoking, dyslipidemia, microalbuminuria), as well as all patients with clinical manifestations of atherosclerosis as secondary prevention.
♦ Screening and treatment of coronary artery disease. Stress tests to exclude coronary artery disease are indicated for patients with symptoms of cardiovascular diseases, as well as when pathology is detected by ECG.
Forecast
75% of patients with T2DM and 35% of patients with T1DM die from cardiovascular diseases. Approximately 50% of patients with T2DM die from complications of coronary artery disease, 15% from cerebral thromboembolism. Mortality from myocardial infarction in people with diabetes exceeds 50%.
7.8.2. Diabetic retinopathy
Diabetic retinopathy(DR) - microangiopathy of the retinal vessels, characterized by the development of microaneurysms, hemorrhages, exudative changes and proliferation of newly formed vessels, leading to partial or complete loss of vision (Table 7.17).
Etiology
The main etiological factor in the development of DR is chronic hyperglycemia. Other factors (arterial hypertension, dyslipidemia, smoking, pregnancy, etc.) are of less importance.
Pathogenesis
The main links in the pathogenesis of DR are:
Microangiopathy of retinal vessels, leading to narrowing of the lumen of blood vessels with the development of hypoperfusion;
Vascular degeneration with the formation of microaneurysms;
Progressive hypoxia, stimulating vascular proliferation and leading to fatty degeneration and deposition of calcium salts in the retina;
Table 7.17. Diabetic retinopathy
 microinfarctions with exudation, leading to the formation of soft “cotton-wool spots”;
microinfarctions with exudation, leading to the formation of soft “cotton-wool spots”;
Deposition of lipids with the formation of dense exudates;
Proliferation of proliferating vessels in the retina with the formation of shunts and aneurysms, leading to dilation of the veins and worsening retinal hypoperfusion;
The phenomenon of stealing with further progression of ischemia, which causes the formation of infiltrates and scars;
Retinal detachment as a result of its ischemic disintegration and the formation of vitreoretinal tractions;
Vitreous hemorrhages resulting from hemorrhagic infarctions, massive vascular invasion and rupture of aneurysms;
Proliferation of the vessels of the iris (diabetic rubeosis), leading to the development of secondary glaucoma;
Maculopathy with retinal edema.
Epidemiology
DR is the most common cause of blindness among the working population of developed countries, and the risk of developing blindness in patients with DM is 10-20 times higher than in the general population. At the time of diagnosis of T1DM, DR is not detected in almost any of the patients; after 5 years, the disease is detected in 8% of patients, and with thirty years of diabetes experience - in 98% of patients. At the time of diagnosis of T2DM, DR is detected in 20-40% of patients, and among patients with fifteen years of T2DM experience - in 85%. In DM-1, proliferative retinopathy is relatively more common, and in DM-2 - maculopathy (75% of maculopathy cases).
Clinical manifestations
According to the generally accepted classification, there are 3 stages of DR
(Table 7.18).
Diagnostics
A complete ophthalmological examination, including direct ophthalmoscopy with retinal photography, is indicated for patients with T1DM 3-5 years after the onset of the disease, and for patients with T2DM immediately after its diagnosis. In the future, such studies must be repeated annually.
Table 7.18. Classification of diabetic retinopathy
 Differential diagnosis
Differential diagnosis
Other eye diseases in patients with diabetes.
Treatment
The basic principle of treatment of diabetic retinopathy, as well as other late complications, is optimal compensation of diabetes. The most effective method for treating diabetic retinopathy and preventing blindness is laser photocoagulation. Purpose
 Rice. 7.11. Diabetic retinopathy:
Rice. 7.11. Diabetic retinopathy:
a) non-proliferative; b) preproliferative; c) proliferative
laser photocoagulation is the cessation of the functioning of newly formed vessels, which pose the main threat of the development of such severe complications as hemophthalmos, traction retinal detachment, iris rubeosis and secondary glaucoma.
Forecast
Blindness is recorded in 2% of patients with diabetes (3-4% of patients with type 1 diabetes and 1.5-2% of patients with type 2 diabetes). The estimated incidence of new cases of blindness associated with DR is 3.3 cases per 100,000 population per year. In type 1 diabetes, reducing HbA1c to 7.0% leads to a reduction in the risk of developing DR by 75% and a reduction in the risk of progression of DR by 60%. In type 2 diabetes, a 1% reduction in HbA1c leads to a 20% reduction in the risk of developing DR.
7.8.3. Diabetic nephropathy
Diabetic nephropathy(DNF) is defined as albuminuria (more than 300 mg of albumin per day or proteinuria more than 0.5 g of protein per day) and/or decreased renal filtration function in persons with diabetes in the absence of urinary infections, heart failure, or other kidney diseases. Microalbuminuria is defined as albumin excretion of 30-300 mg/day or 20-200 mcg/min.
Etiology and pathogenesis
The main risk factors for DNF are duration of diabetes, chronic hyperglycemia, arterial hypertension, dyslipidemia, and kidney disease in parents. In DNF, the first thing that is affected is glomerular apparatus kidneys
1. One of the possible mechanisms by which hyperglycemia promotes the development of glomerular damage, is the accumulation of sorbitol due to the activation of the polyol pathway of glucose metabolism, as well as a number of advanced glycation end products.
2. Hemodynamic disorders, namely intraglomerular hypertension(increased blood pressure inside the glomeruli of the kidney) is an essential component of pathogenesis
The cause of intraglomerular hypertension is a violation of the tone of the arterioles: dilation of the afferent and narrowing of the efferent.
Table 7.19. Diabetic nephropathy
 This, in turn, occurs under the influence of a number of humoral factors, such as angiotensin-2 and endothelin, as well as due to a violation of the electrolyte properties of the glomerular basement membrane. In addition, intraglomerular hypertension is promoted by systemic hypertension, which is detected in the majority of patients with DNF. Due to intraglomerular hypertension, damage to the basement membranes and filtration pores occurs,
This, in turn, occurs under the influence of a number of humoral factors, such as angiotensin-2 and endothelin, as well as due to a violation of the electrolyte properties of the glomerular basement membrane. In addition, intraglomerular hypertension is promoted by systemic hypertension, which is detected in the majority of patients with DNF. Due to intraglomerular hypertension, damage to the basement membranes and filtration pores occurs,
through which traces begin to penetrate (microalbuminuria), and then significant amounts of albumin (proteinuria). Thickening of the basement membranes causes a change in their electrolyte properties, which in itself leads to more albumin entering the ultrafiltrate even in the absence of a change in the size of the filtration pores.
3. Genetic predisposition. Arterial hypertension occurs with increased frequency in relatives of patients with DNF. There is evidence of a connection between DNF and ACE gene polymorphism. Microscopically, with DNF, thickening of the glomerular basement membranes, expansion of the mesangium, as well as fibrous changes in the afferent and efferent arterioles are detected. At the final stage, which clinically corresponds to chronic renal failure (CRF), focal (Kimmelstiel-Wilson) and then diffuse glomerulosclerosis are determined.
Epidemiology
Microalbuminuria is detected in 6-60% of patients with T1DM 5-15 years after its manifestation. DNF is detected in 35% of people with T1DM, more often in men and in people who developed T1DM before the age of 15 years. In T2DM, DNF develops in 25% of Caucasians and 50% of Asians. The overall prevalence of DNF in T2DM is 4-30%.
Clinical manifestations
A relatively early clinical manifestation that is indirectly associated with DNF is arterial hypertension. Other clinically obvious manifestations are late. These include manifestations of nephrotic syndrome and chronic renal failure.
Diagnostics
Screening for DNF in people with diabetes involves annual testing for microalbuminuria for DM-1, 5 years after the manifestation of the disease, and for DM-2, immediately after its detection. In addition, at least annual creatinine levels are required to calculate glomerular filtration rate (GFR). GFR can be calculated using various formulas, for example, the Cockcroft-Gault formula:
For men: a = 1.23 (normal GFR 100 - 150 ml/min) For women: a = 1.05 (normal GFR 85 - 130 ml/min)
In the initial stages of DNF, an increase in GFR may be detected, which gradually decreases as chronic renal failure develops. Microalbuminuria begins to be detected 5-15 years after the manifestation of DM-1; with T2DM in 8-10% of cases it is detected immediately after its detection, probably due to the long asymptomatic course of the disease before diagnosis. The peak development of overt proteinuria or albuminuria in T1DM occurs between 15 and 20 years after onset. Proteinuria indicates irreversibility DNF, which sooner or later will lead to chronic renal failure. Uremia develops on average 7-10 years after the onset of overt proteinuria. It should be noted that GFR does not correlate with proteinuria.
Differential diagnosis
Other causes of proteinuria and renal failure in people with diabetes. In most cases, DNF is combined with arterial hypertension, diabetic retinopathy or neuropathy, in the absence of which the differential diagnosis should be especially careful. In 10% of cases with DM-1 and in 30% of cases with DM-2, proteinuria is not associated with DNF.
Treatment
♦ Basic conditions of primary and secondary prevention
DNF are compensation of diabetes and maintaining normal systemic blood pressure. In addition, primary prevention of DNF involves reducing the consumption of protein foods - less than 35% of daily calories.
♦ At stages microalbuminuria And proteinuria patients are prescribed ACE inhibitors or angiotensin receptor blockers. With concomitant arterial hypertension, they are prescribed in antihypertensive doses, if necessary in combination with other antihypertensive drugs. With normal blood pressure, these drugs are prescribed in doses that do not lead to the development of hypotension. Both ACE inhibitors (for DM-1 and DM-2) and angiotensin receptor blockers (for DM-2) help prevent the transition of microalbuminuria to proteinuria. In some cases, against the background of this therapy in combination with compensation of diabetes by other parameters, microalbuminuria is eliminated. In addition, starting from the stage of microalbuminuria, it is necessary
reducing protein intake to less than 10% of daily calories (or less than 0.8 grams per kg of weight) and salt to less than 3 grams per day.
♦ At the stage chronic renal failure, as a rule, adjustment of glucose-lowering therapy is required. Most patients with T2DM need to be switched to insulin therapy, since the accumulation of TSP carries the risk of developing severe hypoglycemia. Most patients with T1DM experience a decrease in insulin requirements, since the kidney is one of the main sites of insulin metabolism. When the serum creatinine level increases to 500 μmol/L or more, it is necessary to raise the question of preparing the patient for extracorporeal (hemodialysis, peritoneal dialysis) or surgical (kidney transplantation) treatment method. Kidney transplantation is indicated when the creatinine level reaches 600-700 µmol/l and the glomerular filtration rate decreases to less than 25 ml/min, hemodialysis - 1000-1200 µmol/l and less than 10 ml/min, respectively.
Forecast
50% of patients with type 1 diabetes and 10% of patients with type 2 diabetes who have proteinuria develop chronic renal failure over the next 10 years. 15% of all deaths in patients with type 1 diabetes under 50 years of age are associated with chronic renal failure due to DNF.
7.8.4. Diabetic neuropathy
Diabetic neuropathy(DNE) is a combination of syndromes of damage to the nervous system, which can be classified depending on the predominant involvement of its various parts in the process (sensorimotor, autonomic), as well as the prevalence and severity of the damage (Table 7.20).
I. Sensorimotor neuropathy:
Symmetrical;
Focal (mononeuropathy) or polyfocal (cranial, proximal motor, mononeuropathy of the limbs and trunk).
II. Autonomic (autonomic) neuropathy:
Cardiovascular (orthostatic hypotension, cardiac denervation syndrome);
Gastrointestinal (gastric atony, biliary dyskinesia, diabetic enteropathy);
Urogenital (with dysfunction of the bladder and sexual function);
The patient's ability to recognize hypoglycemia is impaired;
Impaired pupil function;
Dysfunction of the sweat glands (distal anhidrosis, hyperhidrosis when eating).
Table 7.20. Diabetic neuropathy
 Etiology and pathogenesis
Etiology and pathogenesis
The main cause of DNE is hyperglycemia. Several mechanisms of its pathogenesis are suggested:
Activation of the polyol pathway of glucose metabolism, resulting in the accumulation of sorbitol, fructose and a decrease in the content of myoinositol and glutathione in nerve cells. This, in turn, leads to the activation of free radical processes and a decrease in the level of nitric oxide;
Non-enzymatic glycosylation of membrane and cytoplasmic proteins of nerve cells;
Microangiopathy vasa nerve which leads to a slowdown in capillary blood flow and nerve hypoxia.
Epidemiology
The prevalence of DNE in both types of diabetes is about 30%. With T1DM, after 5 years from the onset of the disease, it begins to be detected in 10% of patients. The incidence of new cases of DNE in T2DM is about 6% of patients per year. The most common variant is distal symmetric sensorimotor DNE.
Clinical manifestations
Sensorimotor DAY manifests itself as a complex of motor and sensory disorders. A common symptom of the distal form of DNE are paresthesia, which are manifested by a feeling of “crawling goosebumps”, numbness. Patients often complain of chilly feet, although they remain warm to the touch, which is a sign that distinguishes polyneuropathy from ischemic changes, when the feet are cold to the touch. An early manifestation of sensory neuropathy is a violation of vibration sensitivity. Characteristic is the “restless legs” syndrome, which is a combination of nighttime paresthesia and increased sensitivity. Leg pain most often bothered at night, and sometimes the patient cannot bear the touch of a blanket. In a typical case, pain, in contrast to that in occlusive arterial diseases, may decrease with walking. Over the years, the pain may spontaneously stop due to the death of small nerve fibers responsible for pain sensitivity. Hypoesthesia manifested by loss of sensitivity in the “stockings” and “gloves” manner. Violation of deep, proprioceptive sensitivity leads to impaired coordination and difficulty moving (sensory ataxia). The patient complains of “alien legs”, a feeling of “standing on cotton wool”. Violation of trophic innervation leads to degenerative changes in the skin, bones and tendons. Impaired pain sensitivity leads to frequent, unnoticed by the patient, microtraumas of the feet, which easily become infected. Impaired coordination and walking leads to a non-physiological redistribution of the load on the joints of the foot. As a result, the anatomical relationships in the musculoskeletal system of the leg are disrupted.
The arch of the foot is deformed, swelling, fractures, and chronic purulent processes develop (see section 7.8.5).
There are several forms of autonomous daylight. Cause cardiovascular form- disruption of the innervation of the cardiopulmonary complex and large vessels. The vagus nerve is the longest nerve, and therefore is affected earlier than others. As a result of the predominance of sympathetic influences, resting tachycardia. An inadequate response to orthostasis appears orthostatic hypotension and syncope. Autonomic denervation of the pulmonary-cardiac complex leads to the absence of heart rate variability. Autonomic neuropathy is associated with an increased prevalence of silent myocardial infarction among patients with diabetes.
Symptoms gastrointestinal form DNE are gastroparesis with slow or, conversely, rapid gastric emptying, which can create difficulties in the selection of insulin therapy, since the time and volume of carbohydrate absorption varies indefinitely; esophageal atony, reflux esophagitis, dysphagia; watery diarrhea. For urogenital form DNE is characterized by atony of the ureters and bladder, leading to a tendency to urinary infections; erectile dysfunction (about 50% of patients with diabetes); retrograde ejaculation.
Other possible manifestations of vegetative DNE are impaired ability to recognize hypoglycemia, impaired pupil function, impaired sweat gland function (anhidrosis), and diabetic amyotrophy.
Diagnostics
Neurological examination of patients with diabetes should be carried out annually. At a minimum, it involves conducting tests aimed at identifying distal sensorimotor neuropathy. This is done by assessing vibration sensitivity using a graduated tuning fork, tactile sensitivity using a monofilament, as well as temperature and pain sensitivity. According to indications, the state of the autonomic nervous system is studied: to diagnose insufficiency of parasympathetic innervation of the heart, a number of functional tests are used, such as measuring heart rate during deep breathing with assessment of variability
heart rate and Valsalva maneuver; To diagnose insufficiency of sympathetic innervation of the heart, an orthostatic test is used.
Differential diagnosis
Neuropathies of other origins (alcoholic, uremic, with B 12-deficiency anemia, etc.). The diagnosis of dysfunction of a particular organ as a result of autonomic neuropathy is established only after excluding organ pathology.
Treatment
1. Optimization of glucose-lowering therapy.
2. Foot care (see clause 7.8.5).
3. The effectiveness of neurotropic drugs (α-lipoic acid) is not confirmed in all studies.
4. Symptomatic therapy (pain relief, sildenafil for erectile dysfunction, fludrocortisone for orthostatic hypotension, etc.).
Forecast
At the initial stages, DNE can be reversible against the background of persistent compensation of diabetes. DNE is detected in 80% of patients with ulcerative lesions and is the main risk factor for leg amputation
7.8.5. Diabetic foot syndrome
Diabetic foot syndrome(SDS) is a pathological condition of the foot in diabetes, arising against the background of damage to peripheral nerves, skin and soft tissues, bones and joints and manifested by acute and chronic ulcers, osteoarticular lesions and purulent-necrotic processes (Table 7.21).
Etiology and pathogenesis
The pathogenesis of DFS is multicomponent and is represented by a combination of neuropathic and perfusion disorders with a pronounced tendency to infection. Based on the predominance of one or another of the listed factors in the pathogenesis, 3 main forms are distinguished
Table 7.21. Diabetic foot syndrome
 I. Neuropathic form(60-70 %):
I. Neuropathic form(60-70 %):
Without osteoarthropathy;
With diabetic osteoarthropathy.
II. Neuroischemic (mixed) form(15-20 %).
III. Ischemic form(3-7 %).
Neuropathic form of SDS. In diabetic neuropathy, the distal portions of the longest nerves are primarily affected. Long-term deficiency of trophic impulses leads to hypotrophy of the skin, bones, ligaments, tendons and muscles. The result of hypotrophy of connective structures is deformation of the foot with non-physiological redistribution of the supporting load and its excessive increase in certain areas. In these places, for example in the area of the projection of the heads of the metatarsal bones, thickening of the skin and the formation of hyperkeratoses are noted. Constant pressure on these areas leads to inflammatory autolysis of the underlying soft tissue, which creates the preconditions for the formation of an ulcerative defect. As a result of atrophy and impaired sweating, the skin becomes dry and easily cracks. Due to decreased pain sensitivity, the patient often does not pay attention to the changes occurring. He cannot promptly detect the inconvenience of shoes, which leads to the formation of abrasions and calluses, and does not notice the introduction of foreign bodies or small wounds in places of cracking. The situation is aggravated by a violation of deep sensitivity, manifested in gait disturbances and incorrect positioning of the legs. Most often, the ulcerative defect is infected with staphylococci, streptococci, and intestinal bacteria; anaerobic flora often joins. Neuropathic osteoarthropathy is the result of pronounced dystrophic changes in the osteoarticular apparatus of the foot (osteoporosis, osteolysis, hyperostosis).
Ischemic form of SDS is a consequence of atherosclerosis of the arteries of the lower extremities, leading to disruption of the main blood flow, i.e. is one of the variants of diabetic macroangiopathy.
Epidemiology
DDS is observed in 10-25%, and according to some data, in one form or another in 30-80% of patients with diabetes. In the United States, the annual cost of treating patients with diabetes mellitus with DDS is $1 billion.
Clinical manifestations
At neuropathic form SDS distinguishes two most common types of lesions: neuropathic ulcer and osteoarthropathy (with the development
 Rice. 7.12. Neuropathic ulcer in diabetic foot syndrome
Rice. 7.12. Neuropathic ulcer in diabetic foot syndrome
 Rice. 7.13. Charcot joint in diabetic foot syndrome
Rice. 7.13. Charcot joint in diabetic foot syndrome
Charcot joint). Neuropathic ulcers, As a rule, they are localized in the area of the sole and interdigital spaces, i.e. on areas of the foot experiencing the greatest pressure (Fig. 7.12).
Destructive changes in the ligamentous system of the foot can progress over many months and lead to severe bone deformation - diabetic osteoarthropathy and formation Charcot joint, in this case, the foot is figuratively compared to a “bag of bones”
At ischemic form of SDS
the skin on the feet is cold, pale or cyanotic; less often it has a pinkish-red tint due to dilation of superficial capillaries in response to ischemia. Ulcerative defects occur as acral necrosis - on the tips of the fingers, the marginal surface of the heels (Fig. 7.14).
The pulse in the arteries of the foot, popliteal and femoral arteries is weakened or not palpable.
In typical cases, patients complain of “intermittent claudication.” The severity of ischemic limb damage is determined by three main factors: the severity of the stenosis, the development of collateral blood flow, and the state of the blood coagulation system.
Diagnostics
An examination of the legs of a patient with diabetes should be performed every time during a visit to the doctor, at least once every six months. Diagnosis of SDS includes:
 Rice. 7.14. Acral necrosis in the ischemic form of diabetic foot syndrome
Rice. 7.14. Acral necrosis in the ischemic form of diabetic foot syndrome
Examination of the feet;
Assessment of neurological status - various types of sensitivity, tendon reflexes, electromyography;
Assessment of the state of arterial blood flow - angiography, Doppler ultrasound, Doppler sonography;
X-ray of feet and ankle joints;
Bacteriological examination of wound discharge.
Differential diagnosis
It is carried out with wound processes on the feet of a different origin, as well as other occlusive diseases of the vessels of the lower extremities and pathology of the joints of the foot. In addition, it is necessary to differentiate the clinical forms of DFS (Table 7.22).
Treatment
Treatment neuropathically infected The VTS form includes a set of the following activities:
Optimization of compensation for diabetes usually involves increasing the dose of insulin, and in case of diabetes-2 – switching to it;
Systemic antibiotic therapy;
Complete unloading of the foot (this can lead to healing of ulcers that have existed for years within a few weeks);
Local treatment of the wound with removal of areas of hyperkeratosis;
Foot care, proper selection and wearing of special shoes. Timely conservative therapy allows
avoid surgery in 95% of cases.
Table 7.22. Differential diagnosis of clinical forms of DFS
 Treatment ischemic VTS forms include:
Treatment ischemic VTS forms include:
Optimization of compensation for diabetes usually involves increasing the dose of insulin, and in case of diabetes-2 – switching to it;
In the absence of ulcerative-necrotic lesions, ergotherapy (1-2 hours of walking a day, promoting the development of collateral blood flow);
Revascularization operations on affected vessels;
Conservative therapy: anticoagulants, aspirin (up to 100 mg/day), if necessary, fibrinolytics, prostaglandin E1 and prostacyclin preparations.
With the development of extensive purulent-necrotic lesions in all types of DFS, the question of amputation is raised.
Forecast
From 50 to 70% of the total number of leg amputations performed are among patients with diabetes. Leg amputations in patients with diabetes occur 20-40 times more often than in people without diabetes.
7.9. DIABETES AND PREGNANCY
Gestational diabetes mellitus(GDM) is a disorder of glucose tolerance first identified during pregnancy (Table 7.23). This definition does not exclude the possibility that the pathology of carbohydrate metabolism could precede pregnancy. GDM should be distinguished from situations when a woman with previously diagnosed diabetes (due to age, more often T1DM) becomes pregnant.
Etiology and pathogenesis
In GDM they are similar to those in T2DM. High levels of ovarian and placental steroids, as well as an increase in the production of cortisol by the adrenal cortex, lead to the development of physiological insulin resistance during pregnancy. The development of GDM is associated with the fact that insulin resistance, which naturally develops during pregnancy, and, consequently, the increased need for insulin in predisposed individuals exceeds the functional capacity of β-cells of the pancreas. After childbirth, with the return of hormonal and metabolic relationships to the original level, it usually goes away.
Table 7.23. Gestational diabetes mellitus
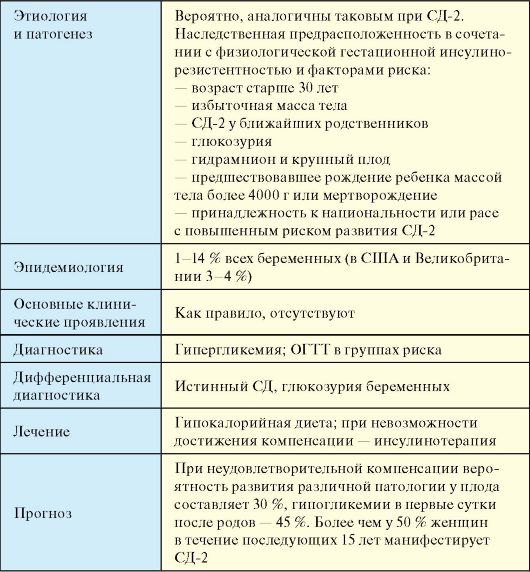 GDM usually develops in the mid-2nd trimester, between 4 and 8 months of pregnancy. The vast majority of patients are overweight and have a history of diabetes mellitus-2. Risk factors for developing GDM, as well as groups of women with a low risk of developing GDM are given in Table. 7.24.
GDM usually develops in the mid-2nd trimester, between 4 and 8 months of pregnancy. The vast majority of patients are overweight and have a history of diabetes mellitus-2. Risk factors for developing GDM, as well as groups of women with a low risk of developing GDM are given in Table. 7.24.
Table 7.24. Risk factors for developing gestational diabetes mellitus
 Maternal hyperglycemia leads to hyperglycemia in the child's circulatory system. Glucose easily penetrates the placenta and continuously passes to the fetus from the mother's blood. Active transport of amino acids and transfer of ketone bodies to the fetus also occur. In contrast, insulin, glucagon and maternal free fatty acids do not enter the fetal blood. In the first 9-12 weeks of pregnancy, the fetal pancreas does not yet produce its own insulin. This time corresponds to the phase of fetal organogenesis when, with constant hyperglycemia, various developmental defects (heart, spine, spinal cord, gastrointestinal tract) can form in the mother. From the 12th week of pregnancy, the fetal pancreas begins to synthesize insulin, and in response to hyperglycemia, reactive hypertrophy and hyperplasia of β-cells of the fetal pancreas develop. Due to hyperinsulinemia, fetal macrosomia develops, as well as inhibition of lecithin synthesis, which explains the high incidence of respiratory distress syndrome in newborns. As a result of β-cell hyperplasia and hyperinsulinemia, there is a tendency to severe and prolonged hypoglycemia.
Maternal hyperglycemia leads to hyperglycemia in the child's circulatory system. Glucose easily penetrates the placenta and continuously passes to the fetus from the mother's blood. Active transport of amino acids and transfer of ketone bodies to the fetus also occur. In contrast, insulin, glucagon and maternal free fatty acids do not enter the fetal blood. In the first 9-12 weeks of pregnancy, the fetal pancreas does not yet produce its own insulin. This time corresponds to the phase of fetal organogenesis when, with constant hyperglycemia, various developmental defects (heart, spine, spinal cord, gastrointestinal tract) can form in the mother. From the 12th week of pregnancy, the fetal pancreas begins to synthesize insulin, and in response to hyperglycemia, reactive hypertrophy and hyperplasia of β-cells of the fetal pancreas develop. Due to hyperinsulinemia, fetal macrosomia develops, as well as inhibition of lecithin synthesis, which explains the high incidence of respiratory distress syndrome in newborns. As a result of β-cell hyperplasia and hyperinsulinemia, there is a tendency to severe and prolonged hypoglycemia.
Epidemiology
Diabetes affects 0.3% of all women of reproductive age, 0.2-0.3% of pregnant women are already initially ill with diabetes, and in 1-14% of pregnancies GDM develops or manifests true diabetes. The prevalence of GDM varies in different populations, for example, in the United States it is detected in approximately 4% of pregnant women (135 thousand cases per year).
Clinical manifestations
Absent in GDM. There may be nonspecific symptoms of decompensated diabetes.
Diagnostics
Determination of fasting blood glucose levels is indicated for all pregnant women as part of a biochemical blood test. Women who are at risk (Table 7.24) are advised to oral glucose tolerance test(OGTT). Many options for its implementation in pregnant women have been described. The simplest of them involves the following rules:
3 days before the examination, the woman eats a normal diet and maintains her usual physical activity;
The test is carried out in the morning on an empty stomach, after an overnight fast of at least 8 hours;
After taking a blood sample on an empty stomach, within 5 minutes the woman drinks a solution consisting of 75 grams of dry glucose dissolved in 250-300 ml of water; The blood glucose level is re-determined after 2 hours.
The diagnosis of GDM is established by the following criteria:
Fasting whole blood glucose (venous, capillary) > 6.1 mmol/l or
Venous blood plasma glucose ≥ 7 mmol/l or
Whole capillary blood glucose or venous blood plasma 2 hours after a load of 75 g glucose ≥ 7.8 mmol/l.
If a woman who is at risk has normal test results, the test is repeated at 24-28 weeks of pregnancy.
Differential diagnosis
GDM and true diabetes; glucosuria in pregnant women.
Treatment
The risk for mother and fetus, as well as approaches to the treatment of diabetes and the features of its control in GDM and true diabetes are the same. Late complications of diabetes during pregnancy can progress significantly, but with high-quality compensation of diabetes there are no indications for termination of pregnancy. A woman suffering from diabetes (usually type 1 diabetes) should plan pregnancy at a young age, when the risk of complications is lowest. If pregnancy is planned, it is recommended to cancel the
reception several months after achieving optimal compensation. Contraindications to pregnancy planning are severe nephropathy with progressive renal failure, severe ischemic heart disease, severe proliferative retinopathy that cannot be corrected, ketoacidosis in early pregnancy (ketone bodies are teratogenic factors).
The purpose of treatment GDM and true diabetes during pregnancy is the achievement of the following laboratory parameters:
Fasting glycemia< 5-5,8 ммоль/л;
Glycemia 1 hour after eating< 7,8 ммоль/л;
Glycemia 2 hours after eating< 6,7 ммоль/л;
Average daily glycemic profile< 5,5 ммоль/л;
HbA1c level with monthly monitoring is the same as in healthy people (4-6%).
With type 1 diabetes, as well as outside pregnancy, a woman should receive intensive insulin therapy, however, it is recommended to assess the level of glycemia during pregnancy 7-8 times a day. If it is impossible to achieve normoglycemic compensation with conventional injections, it is necessary to consider transferring the patient to insulin therapy using an insulin dispenser.
At the first stage treatment of GDM Diet therapy is prescribed, which consists of limiting daily caloric intake to approximately 25 kcal/kg of actual weight, primarily due to easily digestible carbohydrates and fats of animal origin, as well as expanding physical activity. If diet therapy fails to achieve treatment goals, the patient must be prescribed intensive insulin therapy. Any tableted glucose-lowering drugs (TDL) during pregnancy contraindicated. It turns out that about 15% of women need to be switched to insulin therapy.
Forecast
With unsatisfactory compensation of GDM and diabetes during pregnancy, the probability of developing various pathologies in the fetus is 30% (the risk is 12 times higher than in the general population). More than 50% of women who were diagnosed with GDM during pregnancy will develop T2DM over the next 15 years.
